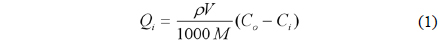During the past decades much attention has been paid to the desulfurization of diesel oil which is important as a source for the fuel cells to prevent the sulfur poisoning of both diesel steam reforming catalyst and electrode of fuel cell. Although alternative desulfurization techniques have been investigated, desulfurization for ultra-low sulfur diesel (ULSD) is still challenged. Therefore, this research focuses on the desulfurization of commercial ULSD for the application to molten carbonate fuel cell (MCFC). Herein, the performances of several kinds of commercial adsorbents based on activated carbons, zeolites, and metal oxides for desulfurization of ULSD were screened. The results showed that metal oxides based materials can feasibly reduce sulfur concentration in ULSD to a level of 0.1 ppmw while activated carbons and zeolites did not reach this level at current conditions.
경유는 연료전지 시스템의 연료원으로써 주목받고 있는 탄화수소 액체연료 중 하나로, 연료개질 촉매와 연료전지의 전극재료를 피독시키는 황화합물을 포함하고 있어 탈황공정이 필요한 것으로 여겨지고 있다. 다양하고도 대안적인 탈황기술이 연구되고 있으나, 초저유황 경유의 탈황 연구는 여전히 부진한 실정이다. 본 연구는 용융탄산염 연료전지 시스템에 응용될 수 있는 원료인 상용초저유황 경유의 탈황에 관한 것이다. 여기서 초저유황의 흡착탈황을 위한 흡착제로 활성탄, 제올라이트, 금속산화물 계 상용흡착제 후보군이 선정되었고 유망한 탈황제를 찾기 위한 스크리닝 평가를 실시하였다. 그 결과 초저유황 경유의 황농도를 0.1 ppmw 수준까지 떨어뜨리기 위한 흡착제 종류로 금속산화물계가 매우 유용하며, 활성탄과 제올라이트 흡착제는 같은 실험조건에서 해당 수준의 황 농도에 이르지 못하는 것으로 나타났다.
The development of the residential applications of fuel cells is an indispensable trend due to the prominent advantages of this technology, which can efficiently generate power in the range of a watt to hundreds of kilowatts with low or zero emissions. Among several kinds of fossil fuels, diesel oil has been considered as one of the promising hydrocarbon fuels for production of hydrogen for use in automotive and portable fuel cells, especially for the proton exchange membrane fuel cell (PEMFC) and solid oxide fuel cell (SOFC) because of its high energy density, available existing infrastructure for production, delivery and storage[1]. An example that the lower heating value of diesel is 42.5 MJ/kg while that of methanol is only 19.9 MJ/kg[2]. Diesel fuel, produced by refining crude oil, contains numerous hydrocarbons between C10 to C22 consisting of paraffins, olefins, and aromatics. In general, reforming of diesel fuel is much more complicated and requires much higher temperatures than 700 ℃ for maximum H2 production due to its complex compositions. Diesel is also contaminated with traces of organic sulfur compounds which their removal is considered to be one of the challenges in utilizing diesel fuel for fuel cell applications. Each type of fuel cell operates at different conditions and thus requires fuel feed in different specifications in terms of composition and purity[3]. Table 1 summaries some of those requirements.
The two types of processes that can be used to remove the sulfur-containing compounds from fuels are hydrodesulfurization (HDS) and the adsorptive desulfurization (ADS) process. The selection of a process depends on the type and quantity of sulfurcontaining compounds to be removed. In general, it can be esta-blished that an HDS process is necessary for a heavy feedstock or a feedstock with high sulfur content, whereas ADS process is only efficient in the case of a light feedstock with low sulfur content (< 20 ppmw). The ADS process can be carried out at both low temperature and high temperature. In low temperature ADS processes, the potential adsorbents are activated carbons [4-7], zeolites[8-11], metal organic frameworks (MOFs)[12-14] while those for high temperature ADS are metals impregnated support material[15-17]. Almost all these articles are academic research, and they are tested with model fuel or commercial fuel with high sulfur concentration. In this research, we investigate potential application of commercial adsorbents for desulfurization of ultra low sulfur diesel (ULSD) in Korea for MCFC applications. The goal of this research is that finding the adsorbent reducing sulfur content in ULSD to the lowest value of sulfur 0.1 ppmw which can satisfy the sulfur limit tolerance of MCFC.
[Table 1.] The fuel specifications required by different types of fuel cells[3]
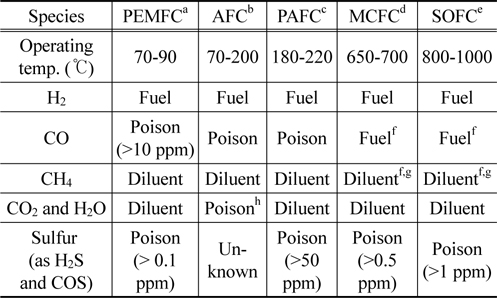
The fuel specifications required by different types of fuel cells[3]
Three samples of virgin activated carbon (AC) are used including AC-1 (Charcoal, Aldrich sigma, code 292251), AC-2 (Coconut shell, Katayama, code 101-1400), and AC-3 (Charcoal, Aldrich sigma, code 242241). Zeolite NaY, HY, HBEA, and HZSM-5 are supplied from Zeolyst Corporation. DP45, DP100, and DP200 are purchased from BASF. Finally, four samples of nickel based adsorbent POS-1, POS-2, POS-3, and POS-4 are received from POSCO Energy Research Center.
2.2. Batch adsorption measurements
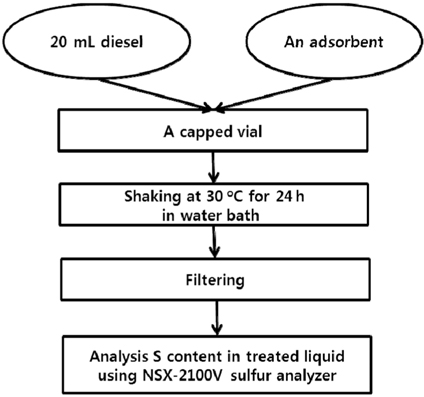
The materials are first tested in batch experiments with a commercial ultra-low sulfur diesel (contain about 6 ppmw S) purchased from a GS Caltex petrol station in Busan, Korea. In a typical experiment, the solid is first activated at the appropriate temperature (about 523 K) and then transferred into a vial of 30 mL. The vial is filled with 20 mL of the commercial diesel, sealed, and placed into a water bath at 303 K. The mixture is kept under vigorous shaking for 24 h, to reach the thermodynamic equilibrium. After filtration, the total sulfur content of the treated diesel is then analyzed by an ultra-trace sulfur analyzer (NSX-2100V, Mitsubishi). Each sample is analyzed three times to estimate error in adsorption capacity. This experiment is repeated for different ratios of solid/liquid. In the case of metal oxide materials, the pretreatment is carried out in hydrogen. All operating procedure for batch experiments can be seen in Figure 1.
The equilibrium adsorption capacity and the sulfur removal efficiency of each adsorbent are calculated by followed equation:
Where:
Qi is equilibrium adsorption capacity (mgS/kg adsorbent)ρ is density of diesel oil (g/mL)V is volume of oil (mL)M is mass of sorbent (g)Co and Ci are initial and final sulfur-concentration in the oil (ppmw)
2.3. Fixed-bed adsorption experiments
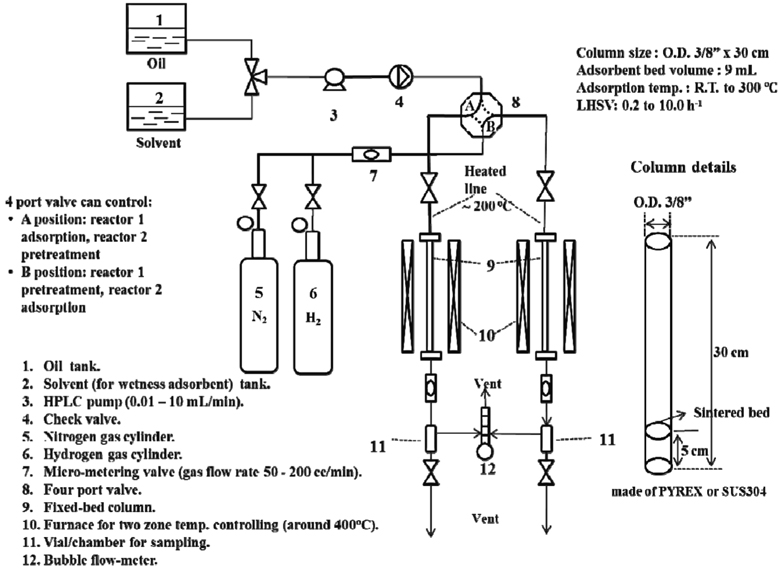
Adsorption experiments are performed at room temperature for activated carbons or zeolites and at 493 K for metal oxide based materials under ambient pressure using a down flow system as seen in Figure 2. About 9 mL of the adsorbent is packed in a glass column having a bed dimension of 7.0 mm i.d. and 300 mm length. The activation of the adsorbent is carried out by heating the adsorbent bed up to 573 K in nitrogen gas (for activated carbon or zeolite materials) or 673 K in hydrogen gas (for metal oxide materials) with a flow rate of 100 mL/min at ambient pressure, and kept at this temperature for 3 h. The furnace temperature is then decreased to the desired adsorption temperature (room temperature or 493 K depend on each adsorbent). The commercial ULSD diesel is fed into the adsorbent column using an HPLC pump, flowed down through the adsorbent bed at a liquid hourly space velocity (LHSV) of 0.34 h-1. The effluent stream of the column is collected periodically each 3 mL for analysis.
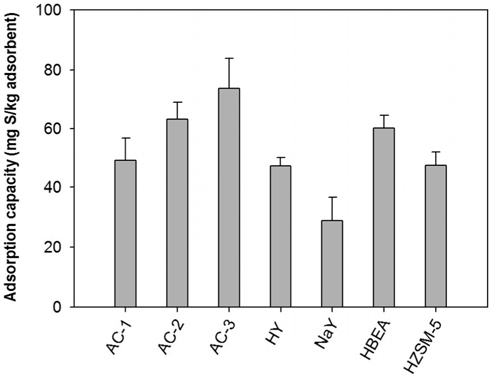
Firstly, the adsorptive performance of several low temperature adsorbents including activated carbons and zeolites is tested in batch mode. The adsorption capacities are shown in Figure 3. Between two groups, activated carbon and zeolite in this study, ACs show a little higher adsorption capacity than those of zeo-lites. ACs group has the order of sulfur uptake as following AC-1 (49.2 mg S/kg adsorbent) < AC-2 (63.1 mg S/kg adsorbent) < AC-3 (73.6 mg S/kg adsorbent) while zeolites group are NaY (28.5 mg S/kg adsorbent) < HY (47.2 mg S/kg adsorbent) < HZSM-5 (47.4 mg S/kg adsorbent) < HBEA (60.2 mg S/kg adsorbent). A higher sulfur uptake of ACs can be explained by their large surface area, large pore volume, and high mesopore volume ratio in comparison with zeolites[18]. Maldonado and Yang[10] also found that the sulfur uptake NaY < HY when they used Y zeolite to remove thiophene in n-octane at both high and low concentration. The good performance of BEA zeolite is also consistent with the results found by Gong et al[11]. It is thought that the wide pore of BEA zeolite enhances to capture big molecular organosulfur compounds in diesel easier than Y or ZSM-5 zeolite.
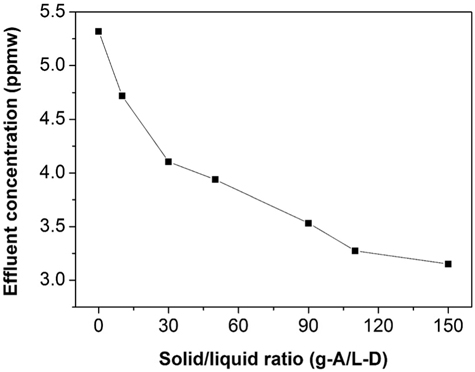
Figure 4 presents the sulfur reduction in the commercial diesel with 5.4 ppmw as a function of the amount of adsorbent used in gram of adsorbent per liter of diesel (g-A/L-D). It is observed that the sulfur concentration in effluent stream decreases as the amount of the adsorbent increase. Using 150 g-A/L-D of BEA zeolite can obtain the total sulfur removal 41%. It also finds that from 110 g-A/L-D the efficiency increases slowly and a difficulty in taking sample occurred due to high loading of solid.
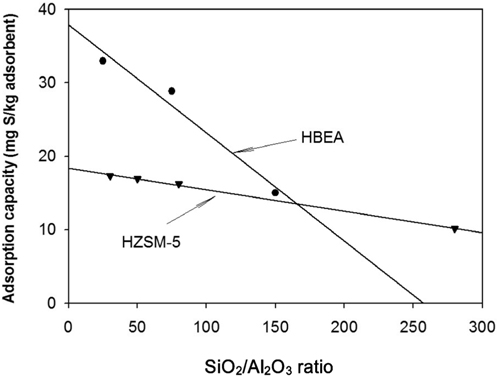
The desulfurization capacity of zeolite depends on a number of factors such as porous structure, acid-base properties which is related to the SiO2/Al2O3 ratio of zeolite framework[10]. Herein, we investigate the desulfurization of sulfur compounds in commercial diesel over HBEA and HZSM-5 zeolites with different SiO2/Al2O3 ratios. Figure 5 shows the sulfur adsorption capacity using BEA zeolite with the SiO2/Al2O3 ratios of 25, 75, and 150 and HZSM-5 with the SiO2/Al2O3 ratios of 30, 50, 80, and 280. The results present that the adsorption capacity decreases with a rising of the SiO2/Al2O3 ratio for both HBEA and HZSM-5 zeolite. However, the decreasing rate is quite different, in which it is fast for HBEA and slow for HZSM-5. It is believed that high SiO2/Al2O3 causes a decrease in acidic property which enhances the sulfur adsorption capacity through acid-base interaction between zeolite and organosulfur compounds as reported by Xu et al[19].
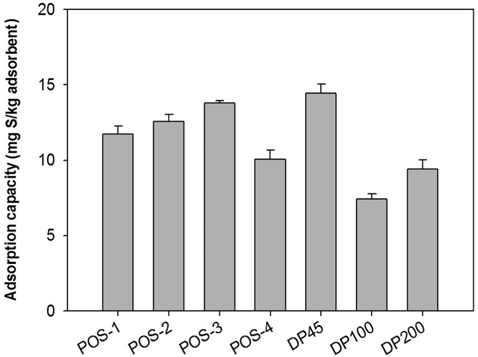
Secondly, the adsorptive performances of commercial adsorbents including nickel-based (POS-1, POS-2, POS-3, and POS-4), copper-based (DP45) and similar zeolite (DP100 and DP200) are tested in batch mode. The adsorption capacities are shown in Figure 6. In these cases, a large amount of adsorbent (150 g-A/L-D) is used to improve the efficiency of desulfurization. Two remarkable samples are POS-3 and DP45 when they can remove 47.5 and 49.5 % sulfur content in treated diesel, respectively.
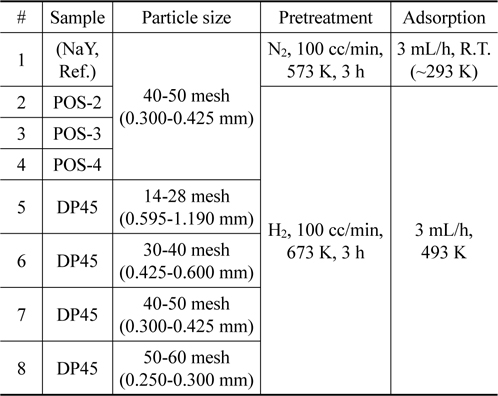
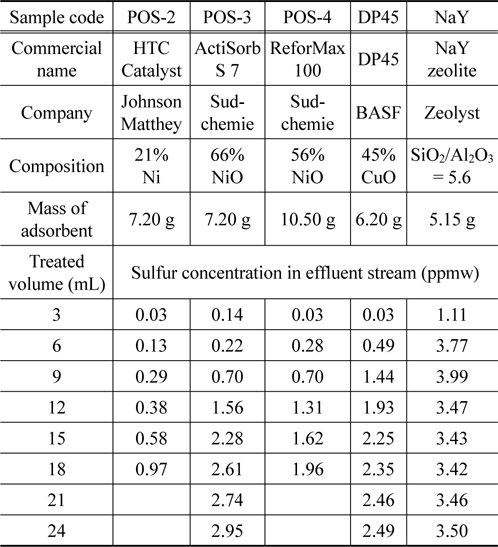
Based on potential adsorbents from batch test, the column test is carried out to evaluate the performance in real application. Experiments are done with six samples including POS-2, POS-3, POS-4 (nickel-based material), DP45 (copper-based material), and NaY zeolite material for comparison. The conditions for pretreatment and adsorption process are listed in Table 2.
[Table 2.] Parameters of experiments for column test
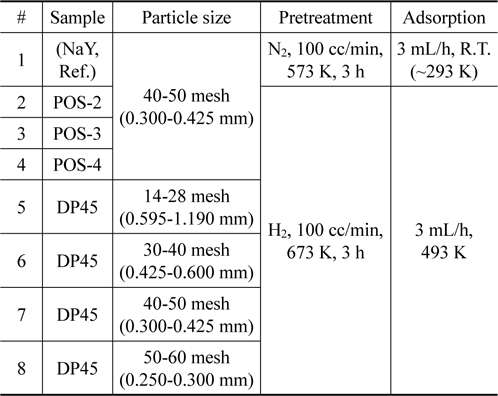
Parameters of experiments for column test
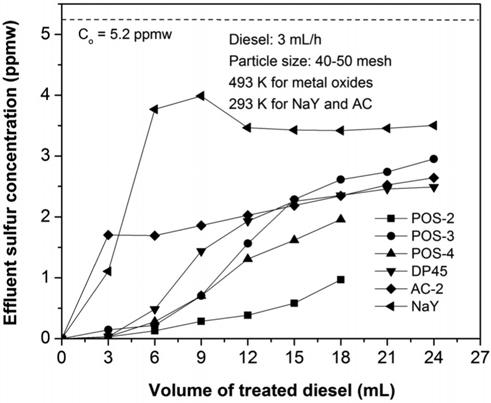
After the adsorbents are activated, commercial ULSD is passed through a fixed-bed column at a flow rate of 3 mL/h and the sulfur concentration of the effluent stream expresses as a function of time. Detail information of the adsorbents and the concentration of the outlet stream are shown in Table 3. Figure 7 presents the breakthrough curves for five adsorbents. The influent concentration is approximate 5.2 ppmw. As can be seen in Figure 7, all samples based on metal oxides can meet the limit level 0.1ppmw while NaY zeolite cannot obtain the goal when they have the first breakthrough point with a higher concentration of 1.0 ppmw. Among metal oxide based adsorbents, POS-2 is the best adsorbent in which it can treat 2.5 mL or 0.67 mL commercial ULSD to reach the level of 1.0 ppmw or 0.1 ppmw, respectively. It is noted that POS-2 contains Ni content of 21 wt% which is much lower than that of POS-3 (66 wt% of NiO) and POS-4 (56 wt% of NiO). The mechanism for sulfur adsorption over metalbased adsorbent such as nickel or copper can be explained the formation of direct S-M bonds or by π complexation[15-17].
[Table 3.] Desulfurization performance of different kinds of adsorbents in column test
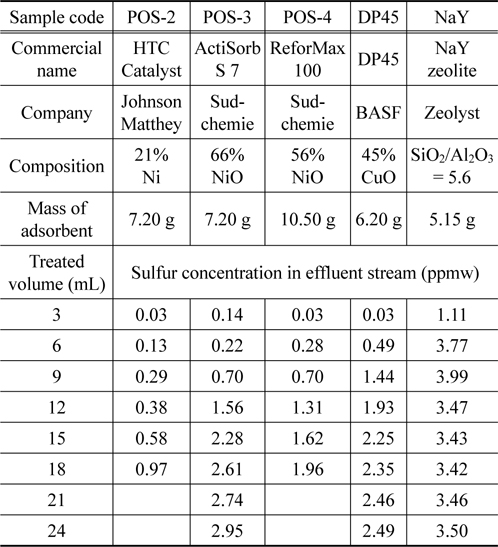
Desulfurization performance of different kinds of adsorbents in column test
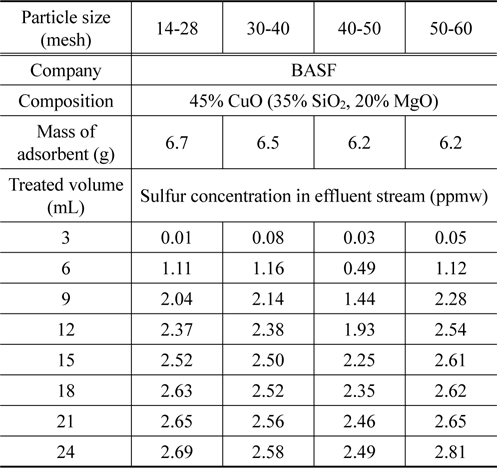
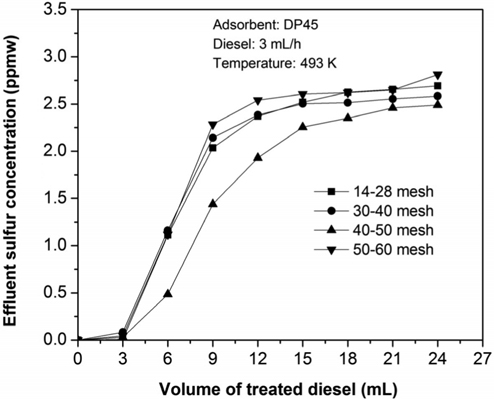
In order to study effect of particle size, four samples of DP45 adsorbent are prepared with different particle size as shown in Table 2. The adsorption process is conducted at 493 K after the adsorbent is pretreated in 100 mL/min of hydrogen at 673 K for 3 h. The results are expressed in Table 4 and Figure 8. As can be seen in Table 4 and Figure 8, the particle size in the range from 14 mesh (1.19 mm) to 60 mesh (0.25 mm) have a nearly the same desulfurization performance, and they can obtain the goal reducing sulfur content below 0.1 ppmw for MCFC application.
[Table 4.] Particle size effect of DP45 adsorbent
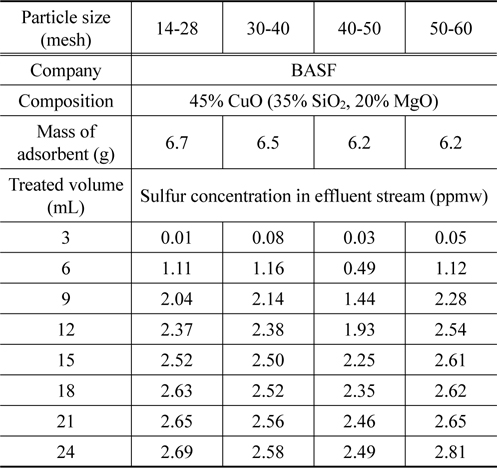
Particle size effect of DP45 adsorbent
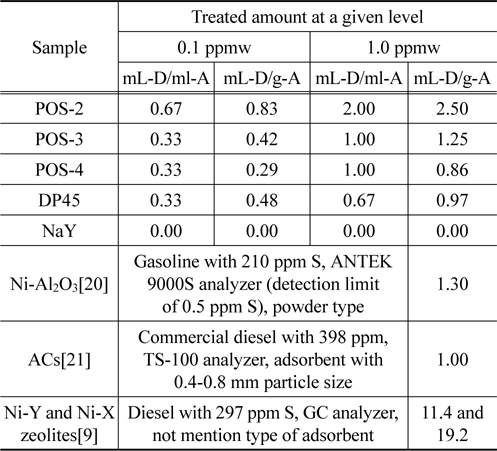
Finally, in order to make an advantage for understanding the results in this study and comparing with other research group, we express adsorption performance via two concepts, namely amount of treated diesel per volume (mL-D/mL-A) and amount of treated diesel per mass of adsorbent (mL-D/g-A) based on two level of sulfur concentration 0.1 and 1.0 ppmw. The summary is shown in Table 5. It is emphasized that it’s possible to desulfurize ULSD to the level of 0.1 ppmw by using adsorption process over commercial metal oxide based adsorbent such as POS-2, POS-3, POS-4, and DP45, in which their performance for desulfurization are nearly the same with the results from the literature using Ni-Al2O3 or AC as the adsorbents. It also finds that NaY is inefficient for desulfurization of ULSD to the level 0.1 ppmw. However, the literature survey indicates that a modification of Y zeolite can improve significantly their desulfurization performance[10]. Based on the structure of sulfur compounds in ULSD, it seems to be difficult to remove these compounds by physisorption when ULSD contains almost all heavy organosulfur which are primarily dibenzothiophene and its derivative[22].
[Table 5.] Summary the performance of ULSD desulfurization over different adsorbents
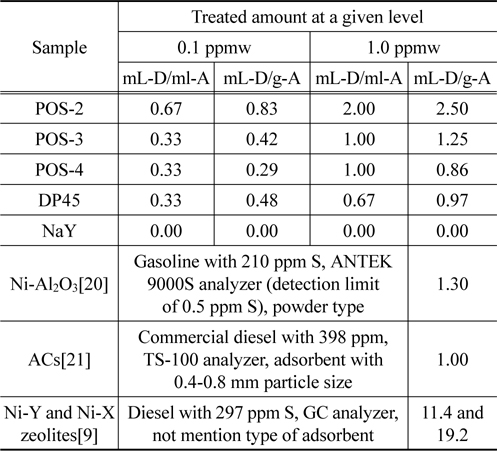
Summary the performance of ULSD desulfurization over different adsorbents
Adsorption of real commercial ULSD over several adsorbent in three typical groups - activated carbons, zeolites, and metal oxides are conducted in both batch and fixed-bed continuous adsorption system. The results reveal that it is possible to reduce sulfur content in commercial ULSD in Korea containing approximate 5 ppmw S to 0.1 ppmw which is the tolerance level for MCFC application. The best adsorbent in this research is POS-2 adsorbent which is a commercial nickel adsorbent. It can reduce the sulfur in 2.5 or 0.67 mL commercial diesel to be lower 1.0 ppmw or 0.1 ppmw concentration, respectively. The results also indicate that activated carbon and zeolite have a lack of performance to meet the goal 0.1 ppmw even though they had a good performance in batch test. The reason is thought that the volume of adsorbent bed is not enough large to obtain a suitable retention time. In future work, based on metal oxide adsorbent (Cu and Ni) our group will modify the reactor type as well as investigate adsorption conditions such as temperature and pressure to improve efficiency of the process to meet the limit level 0.1 ppmw.
![The fuel specifications required by different types of fuel cells[3]](http://oak.go.kr/repository/journal/13188/CJGSB2_2014_v20n1_88_t001.jpg)