Alcohol abuse is a public issue and one of the major causes of liver disease worldwide. This study was aimed at investigating the protective effect of Ganoderma lucidum pharmacopuncture (GLP) against hepatotoxicity induced by acute ethanol (EtOH) intoxication in rats.
Sprague-Dawley (SD) rats were divided into 4 groups of 8 animals each: normal, control, normal saline pharmacopuncture (NP) and GLP groups. The control, NP and GLP groups received ethanol orally. The NP and the GLP groups were treated daily with injections of normal saline and Ganoderma lucidum extract, respectively. The control group received no treatment. The rats in all groups, except the normal group, were intoxicated for 6 hours by oral administration of EtOH (6 g/kg BW).
The same volume of distilled water was administered to the rats in the normal group. Two local acupoints were used: Qimen (LR14) and Taechung (LR3). A histopathological analysis was performed, and the liver function and the activities of antioxidant enzymes were assessed.
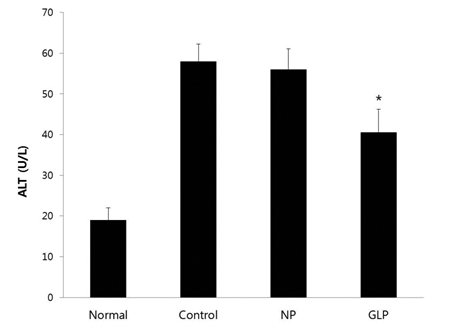
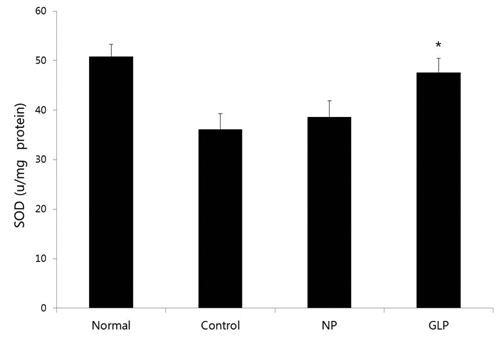
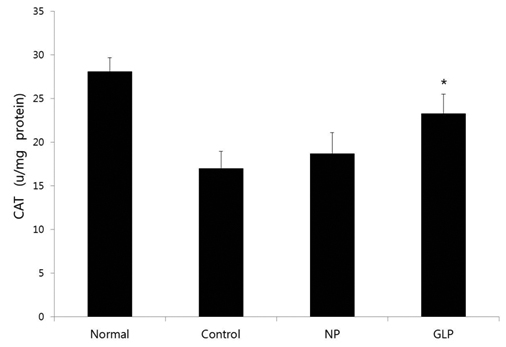
GLP treatment reduced the histological changes due to acute liver injury induced by EtOH and significantly reduced the increase in the alanine aminotransferase (ALT) enzyme; however, it had an insignificant effect in reducing the increase in aspartate aminotransferase (AST) enzyme. It also significantly ameliorated the superoxide dismutase (SOD) and the catalase (CAT) activities.
The present study suggests that GLP treatment is effective in protecting against ethanol-induced acute hepatic injury in SD rats by modulating the activities of ethanol-metabolizing enzymes and by attenuating oxidative stress.
The stomach is a sensitive digestive organ that is susceptible to exogenous pathogens to which it is exposed by the diet [1]. Alcohol abuse is a public issue and one of the major causes of liver disease worldwide. It accounts for a large proportion of the deaths from liver disease [1, 2].
Heavy consumption of alcohol is well known to be associated with liver damage. The close relation between ethanol and liver damage is mainly due to the fact that about 80% of ingested alcohol is metabolized in the liver. Ethanol (EtOH) is metabolized into cytotoxic acetaldehyde by the enzyme alcohol dehydrogenase (ADH) in the liver. Ethanol is metabolized via a series of catabolic metabolic pathways, including complex oxidation reactions in liver tissue [3, 4], and acetaldehyde is oxidized to acetate by aldehyde oxidase or xanthine oxidase, giving rise to reactive oxygen species (ROS) via cytochrome P450 2E1 (CYP 2E1) [5].
Experimental animals exposed to alcohol display biochemical signs of hepatotoxicity and oxidative damage, suggesting a possible role of free radicals in causing some of the toxic effects of alcohol [6]. Excessive alcohol consumption not only enhances ROS generation, but also depletes antioxidants, thus creating a state of oxidative stress that leads to severe liver injury [7-10]. EtOH oxidation generates toxic metabolites and free radicals and induces a state of oxidative stress characterized by an enhancement of lipid peroxidation and a reduction in the activities of endogenous antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase, which contribute to the pathogenesis of alcoholic liver disease (ALD) [11]. These free radicals are capable of damaging many cellular components such as DNA, proteins and lipids. However, several naturally-occurring antioxidant compounds are used to protect against liver diseases both in experimental and clinical situations [12-14].
Pharmacopuncture is an effective therapy in Oriental medicine. A solution extracted from medicinal herbs is injected into acupuncture points according to the condition of the patient [15, 16]. Many studies have proven that mushrooms to be novel and rich sources of bioactive compounds. Among mushrooms,
Adult male Sprague-Dawley (SD) rats (weighing 120 ─ 140 g, 5-weeks old, and housed five per cage) were purchased from SAM-Taco Co. and were used in accordance with the regulations of the local ethics committee of Dong-Eui University for the use and care of animals. The animals were provided with standard food and water ad libitum and were maintained in an animal house at a controlled temperature (22 ± 2℃) with a 12-hours light/dark cycle.
Animals were divided into 4 groups of 8 animals each: normal, control, normal saline pharmacopuncture (NP) and GLP groups. All groups, except the normal group, were intoxicated for 6 hours by oral administration of EtOH (6 g/ kg of body weight (BW)) [21]. Normal group was administered the same volume of distilled water. After 30 minutes of EtOH administration, the NP and the GLP group were treated with injections of normal saline and
All the animals were humanely sacrificed using ether. Immediately after the rats had been sacrificed, small pieces of their livers were harvested and washed with ice-cold saline. Tissue fragments were then fixed in a 10% neutral buffered formalin solution, embedded in paraffin, and used for histopathological examinations. Sections of 5 μm in thickness were cut, deparaffinized, hydrated and stained with hematoxylin and eosin (H&E). The hepatic sections were examined in a blind fashion for all treatments.
Blood of approximately 3 ─ 4 mL was collected and allowed to clot for 30 minutes at room temperature. The serum was separated by centrifugation for 15 minutes and was used to determine marker enzymes, such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT).
In the control, NP, and GLP groups, SD rats were intoxicated by using a single oral administration of EtOH (6 g/ kg BW); however, distilled water was used in the normal group. After 6 hours, the animals were sacrificed, and their livers were rapidly excised and homogenized in 50-mM potassium-phosphate buffer to determine the biochemical parameters. After centrifugation at 10,000 g for 10 minutes at 4℃, the supernatant was used for biochemical determinations of the protein, free-iron, hydrogen-peroxide (H2O2), malondialdehyde (MDA) and antioxidant enzyme activities. In addition, blood was also collected in heparinized tubes. After centrifugation at 3000 g for 15 minutes, plasma was processed to determine the free-iron, H2O2 and transaminase levels.
The SOD activity was determined using a modified epinephrine assay [22]. At alkaline pH, the superoxide anion O2 causes the autoxidation of epinephrine to adrenochrome; while competing with this reaction, SOD decreased the adrenochrome formation. One unit of SOD is defined as the amount of the extract that inhibits the rate of adrenochrome formation by 50%. Enzyme extract was added to a 2-mL reaction mixture containing 10 mL of bovine CAT (0.4 U/mL), 20 mL of epinephrine (5 mg/mL) and a 62.5-mM sodium carbonate/bicarbonate buffer at pH 10.2. Changes in absorbance were recorded at 480 nm. The CAT activity was assayed by measuring the initial rate of H2O2 disappearance at 240 nm. The assay for total SOD was based on its ability to inhibit the oxidation of hydroxylamine by the xanthine–anthine oxidase system.
Data were analyzed by using the Student's
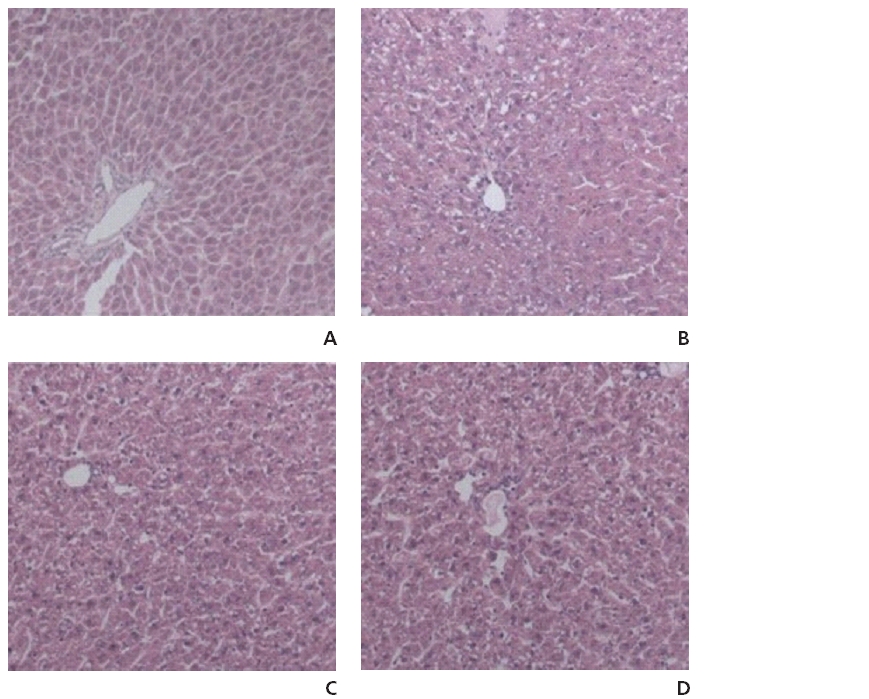
Some results from the histopathological examinations of the liver sections from the experimental rats are shown in (Fig 1) No histopathological alterations and normal histological structures of the central vein and the surrounding hepatocytes were recorded in the liver sections of the rats in the normal group (Fig 1A). The liver sections of rats in the normal group showed hepatic lobules consisting of a central vein surrounded by radiating hepatocytes separated by irregular blood sinusoids. On the other hand, the liver sections of rats treated with EtOH showed severe histopathological alterations appearing as centrilobular areas with necrosis and different degenerative changes, such as ballooning, fatty and hydropic degeneration, in association with inflammatory cell infiltration and dilatation in the central vein (Fig 1B). In the NP group, the centrilobular area showed a lower grade of degeneration, necrosis and inflammatory cell infiltration compared to the control group (Fig 1C). The GLP group showed a partially-reduced degenerative process and hepatocellular steatosis induced by EtOH treatment (Fig 1D). However, compared to the control group, the changes detected in both the NP and the GLP groups are not considered to be significant.
The SD rats were intoxicated by using a single dose of EtOH (6 g/kg BW) and were then treated with normal saline solution or
We also studied the activities of the hepatic antioxidant enzymes. EtOH intoxication significantly decreased the activities of the hepatic antioxidant enzymes SOD and CAT.
GLP significantly reversed all EtOH-induced decreases in the activities of the antioxidant enzymes SOD and CAT (Figs 4,5)
Ethanol is found in fermented drinks that are widely consumed across the world and causes global health problems. Alcohol consumption is related to more than 60 medical conditions and has been suggested to be responsible for 25% of liver-cancer cases, as well as 32% of liver-cirrhosis cases, worldwide [23, 24]. ALD is a major cause of morbidity and mortality around the world [25]. Ethanol administration causes accumulation of ROS, including superoxides, hydroxyl radicals, and hydrogen peroxide [26]. ROS, in turn, cause lipid peroxidation of cellular membranes, and protein and DNA oxidation, which results in hepatocyte injury [27-29]. Both the formation of pro-oxidants and the depletion of antioxidants caused by ethanol administration result in oxidative stress [30, 31]. Another major consequence of ethanol metabolism is lipid accumulation in the liver. Ethanol metabolism changes the NAD/NADH ratio, which has important consequences for fuel utilization in the liver, favoring the synthesis of fatty acids and inhibiting the oxidation of fatty acids [32, 33].
Pharmacopuncture or herbal acupuncture is a new form of therapy derived from a combination of two traditional therapeutic methods, herbal medicine and acupuncture therapy. Recently, pharmacopuncture therapies in Korea have developed into quite a unique and systematic framework for the diagnosis and treatment of various diseases [34].
First, the present investigation revealed that acute administration of EtOH for 6 hours resulted in a clear hepatotoxicity, as evidenced by the increased plasma AST and ALT (Figs 2,3), the indices of liver injury, and tissue damage revealed by histological observations (Fig 1A). ALD is characterized by morphological changes ranging from hepatic steatosis, inflammation and hepatic necrosis to progressive fibrosis [38, 39].
In this study, the liver sections of the rats treated with EtOH for 6 hours showed microvesicular steatosis and spotty necrosis (Fig 1B).
GLP reduced the histological changes due to acute liver injury induced by EtOH intoxication (Fig 1D). In other words, our data showed that GLP attenuated EtOH-induced acute hepatic-tissue injury. We suggest that GLP prevents the structural degeneration of hepatocytes by decreasing the formation of inflammatory and fibrogenic mediators. Our data also show that GLP treatment eliminates almost all the indications of EtOH-induced liver dysfunction. The high levels of AST and ALT in the plasma compartment are central indications of the degree of damage to the liver caused by EtOH administration [40-42].
Assessment of liver function can be performed by determining the activities of the serum enzymes AST and ALT originally present in high concentrations in the cytoplasm. AST is localized in the heart, brain, skeletal muscles, and liver tissue. ALT is primarily localized in the liver, with lower enzymatic activities being found in skeletal muscles and heart tissue [43].
The serum ALT activity level is the most frequently relied upon as a laboratory indicator of hepatotoxic effects [44], shows infrequent false negative signals of liver histopathologic injury, as well as limited false positive signals, and is considered as the gold standard clinical chemistry marker of liver injury. ALT plays an important role in amino acid metabolism and gluconeogenesis
Increases in the serum ALT and AST enzymatic levels can also arise from extra-hepatic injury, particularly injuries to the skeletal muscles. Newer biomarkers of liver injury, with greater specificity to the liver, could be used in conjunction with ALT for the safety evaluation of developmental compounds in the pharmaceutical industry.
Serum AST activity is considered to be a less specific biomarker of liver function compared to ALT activity. AST is released from damaged myocytes, as well as hepatocytes. In this study, although there were no significant effects on AST enzyme, the treatment with GLP significantly reduced of the ALT enzyme increase induced by administering EtOH for 6 hours, which means that GLP has a protective effect against acute ethanol-induced hepatotoxicity.
Oxidative stress and lipid accumulation play important roles in alcohol-induced liver injury. The predominant factor causing ethanol-associated liver damage is the generation of oxidative stress. Acute alcohol-induced oxidative stress has been widely documented in the liver [45, 46]. EtOH administration provokes an oxidative imbalance through a number of pathways, including the generation of reactive oxygen species via xanthine oxidase [47] and an alteration of defense mechanisms with decreased GPx activity following selenium deficiency [48]. EtOH-induced liver toxicity is also reflected by increased lipoperoxidation, H2O2 and free-iron levels, as well as reduced activities of antioxidant enzymes such as SOD and CAT.
In this study, EtOH intoxication significantly decreased the activities of the hepatic antioxidant enzymes such as SOD and CAT in acute liver injury, and GLP significantly restored the SOD and the CAT activities, which means that GLP treatment protected against lipid peroxidation and reductions in the activities of antioxidant enzymes. These data suggest that GLP treatment may reduce the alcohol-induced oxidative stress in rat livers. Therefore, the antioxidant and anti-lipogenesis activities of GLP make it a promising pharmacopuncture for treating ALD. These results indicate that GLP protects rats against acute EtOH-induced liver injury by reducing oxidative stress and decreasing lipid accumulation.
In summary, apoptotic changes are inhibited by the addition of antioxidants and GLP, suggesting that oxidative stress is involved in the release of cytochrome c, which precedes apoptosis in hepatocytes exposed to ethanol. Therefore, GLP suppresses apoptosis by regulating mitochondrial-damage-mediated endogenous pathways, which could be one of the important mechanisms for preventing alcoholic-induced liver injury. In conclusion, the present study has revealed that GLP protects against EtOH-induced hepatic injury in SD rats by modulating the activities of ethanol-metabolizing enzymes and attenuating oxidative stress.
In this study, GLP was found to reduce the histological changes due to acute liver injury induced by EtOH intoxication. GLP also significantly reduced the increase in the activity of the ALT enzyme caused by acute liver injury and significantly ameliorated activities of SOD and CAT.
![Effect of Ganoderma lucidum pharmacopuncture (GLP) on acute EtOH-induced changes in plasma aspartate aminotransferase (AST).
The plasma AST was increased in all EtOH-treated groups. However, the plasma AST of the GLP group was restored compared with that of the
control group, but the difference was not statistically significant. Data are expressed as means ± SEs [3].](http://oak.go.kr/repository/journal/13156/DHOCBS_2014_v17n3_16_g002.jpg)