Mountain ginseng pharmacopuncture (MGP) is an extract distilled from either mountain cultivated ginseng or mountain wild ginseng. This is the first intravenous injection of pharmacopuncture in Korea. The word intravenous does not discriminate between arteries, veins, and capillaries in Oriental Medicine, but only the vein is used for MGP. The aim of this study is to evaluate the intravenous injection toxicity of MGP through a single-dose test in Sprague-Dawley (SD) rats.
Male and female 6-week-old SD rats were injected intravenously with MGP (high dosage of 20 mL/kg or low dosage of 10 mL/kg). Normal saline was injected into the rats in the control group by using the same method. After the rats has treated, we conducted clinical observations, body-weight measurements and histological observations.
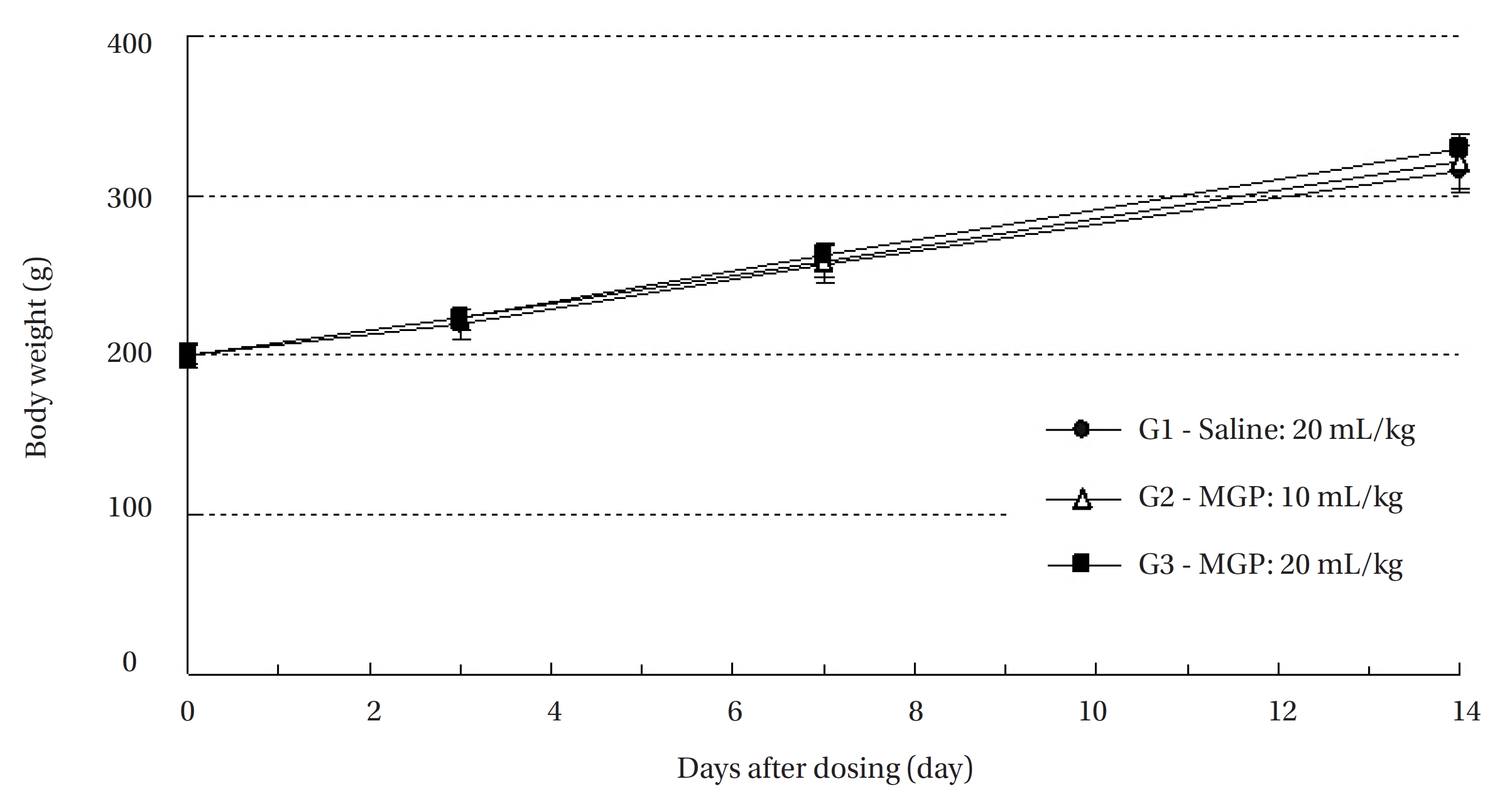
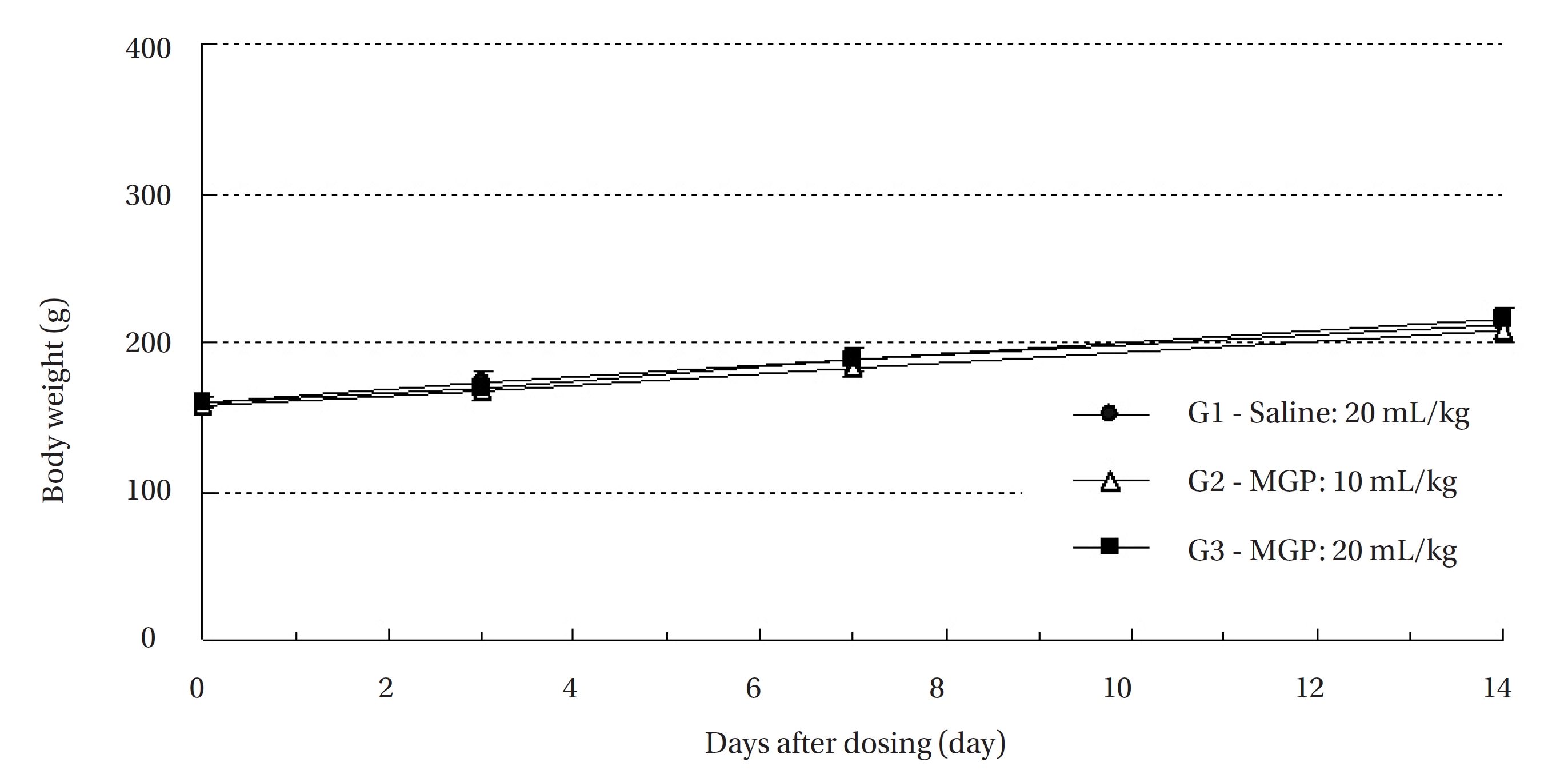
In this study, no mortalities were observed in any of the experimental groups. Also, no significant changes by the intravenous injection of MGP were observed in the body weights, or the histological observations in any of the experimental groups compared to the control group. The lethal dose for intravenous injection of MGP was found to be over 20 mL/kg in SD rats.
Considering that the dosage of MGP generally used each time in clinical practice is about 0.3 mL/kg, we concluded with confidence that MGP is safe pharmacopuncture.
Mountain ginseng pharmacopuncture (MGP) is the first intravenous injection pharmacopuncture using an extract distilled from mountain cultivated ginseng or mountain wild ginseng in korean traditional medicine [1].
Mountain wild ginseng refers to ginseng (
Because authenticity tests of mountain wild ginseng are very difficult and confusing, scientific criteria of authenticity are needed. Thus, an objective diagnostic method that used gene analysis to identify mountain ginseng (MG) was developed. Comparing gene activation between numerous MG samples and regular ginseng, genes only existing in MG, such as p-NRT2, p-rpoC1, p-psbB, and p-GAPDH, were found [3-6]. Because of the high-cost and authenticity issues associated with mountain wild ginseng, mountain cultivated ginseng which is similar to mountain wild ginseng according to the gene analysis, is used for pharmacopuncture [1].
Although continuous clinical studies have been performed to obtain more objective data on the efficacies of MGP, safety tests are still necessary. The aims of this study were to evaluate the safety of MGP, and to conduct single- dose toxicity tests of MGP.
18 Sprague-Dawley (SD) rats of each gender were obtained from a specific pathogen-free facility (Biotoxtech, Oh Chang, Korea) at 5 weeks of age and were used after a week of quarantine and acclimatization. The animals were housed in a room maintained at 19.0 ─ 23.2℃ under a relative humidity of 33.0% ─ 59.5%. The room was illuminated with artificial lighting for 07:00 to 19:00 hours and had 10 — 15 air changes per hour. Three animals were housed in suspended stainless-steel wire-mesh cages and were allowed sterilized tap water and commercial rodent chow (Teklad Certified Irradiated Global 18% Protein Rodent Diet 2918C, Harlan Laboratories, Inc., U.S.A.). This study protocol was approved by the institutional Animal Care of Biotoxtech Co. (Oh Chang, Korea).
The process for the manufacturing MGP was performed in a pathogen-free facility (Korean Pharmacopuncture Institute, Seoul, Korea), as described previously [7]. MG of over 10 years which was cultivated from the seeds of mountain wild ginseng, was selected for the MGP. First, we washed MG with running water and dried it in the air-conditioned room. Second, dried MG was powdered in to fine particles (size are below 10 ㎛) and decocted for 2 hours in distilled water. Remnants were then removed, and the decoction was distilled before yielding the desired herbal acupuncture. Then the pharmacopuncture was filtered using 0.1 ㎛ filter paper and was then kept in the container. Finally, the pharmacopuncture was sterilized before being used.
Healthy male and female rats, 5 of each sex were selected and assigned to 1 of 3 groups: control group (normal saline), low-dose group (10 mg/kg), and high-dose group (20 mg/kg). MGP was administered to the rats by intravenous injection in to the caudal vein. The control group was administered an equivalent volume of normal saline (Lot nos. DAJ9074, DAJ9076).
All animals were observed for clinical signs at 30 minutes, 1 hour and 2 hours after the first injection and at 30 minutes, 1 hour, 2 hours, 4 hours and 6 hours after the second injection. Clinical signs were observed daily from the injection day to 14 day after the first injection. The body weight of each rat was measured at the initiation of treatment, and at 3 days, 7 days and 14 days after the injection.
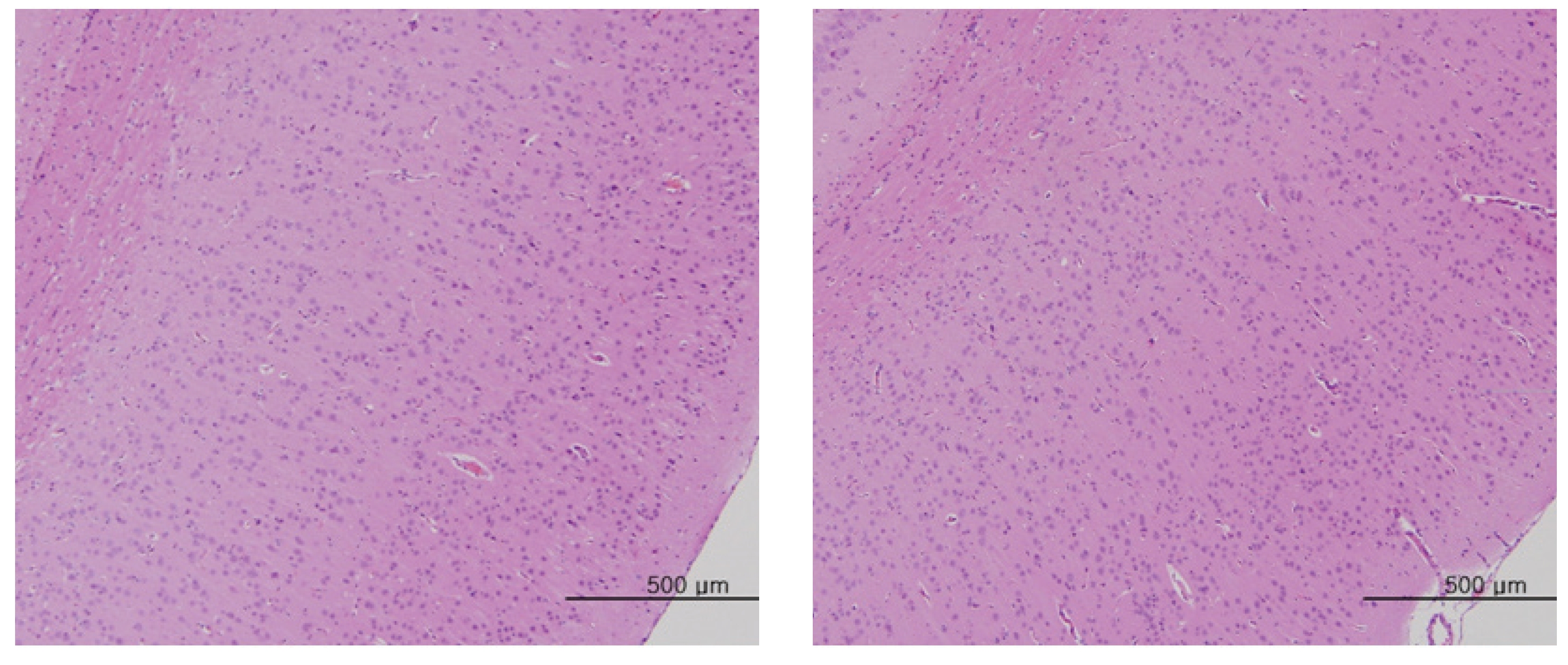
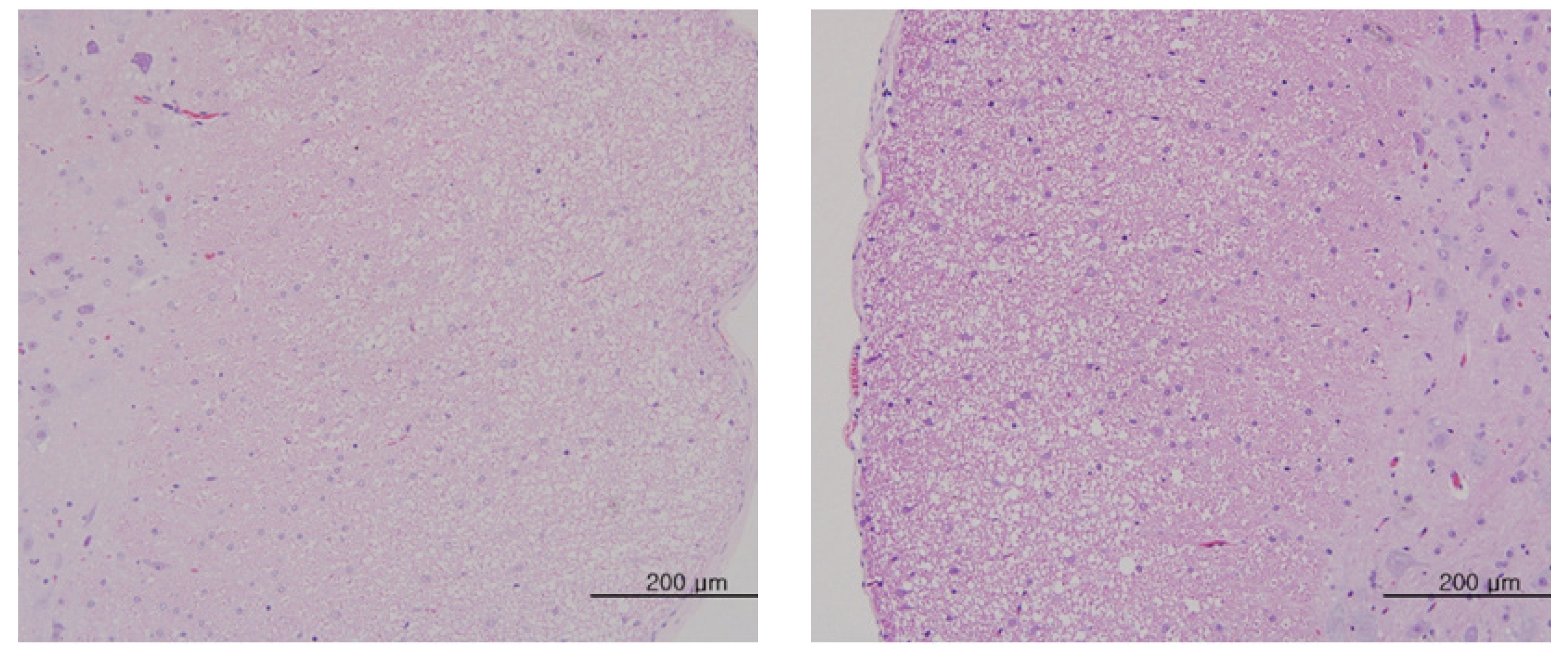
The scheduled day of study termination, all surviving animals were anesthetized by CO2 inhalation. Tissues were obtained from the following organs of all surviving animals and fixed with 10% neutral buffered formalin solution: brain, heart, liver, spleen, kidney, lung, spinal nerves. These tissues were routinely processed, embedded in paraffin and sectioned. The sections were stained with hematoxylin- eosin (H&E) dye for microscopic examination. All organs and tissues taken from all animals were examined microscopically.
Data on body weights were tested by using SAS (version 9.2, SAS Institute Inc, USA). The variance was checked by using the Bartlett test [8]. If the variance was homogeneous, the data were subjected to one-way analysis of variance (ANOVA). If significant changes were observed on the one-way ANOVA, the data were analyzed by using the multiple comparison procedure of Dunnett’s test [9] (
No treatment-related mortalities occurred in SD rats treated with any dose of the MGP. Also, no clinical signs were observed in the male and the female SD rats of the experimental groups during the observation period. Hematuria was observed in 1 of the rats of control groups, but it was not caused by MPG. No significant changes in weight were observed in the experimental groups compared to the control group (Fig 1,2).
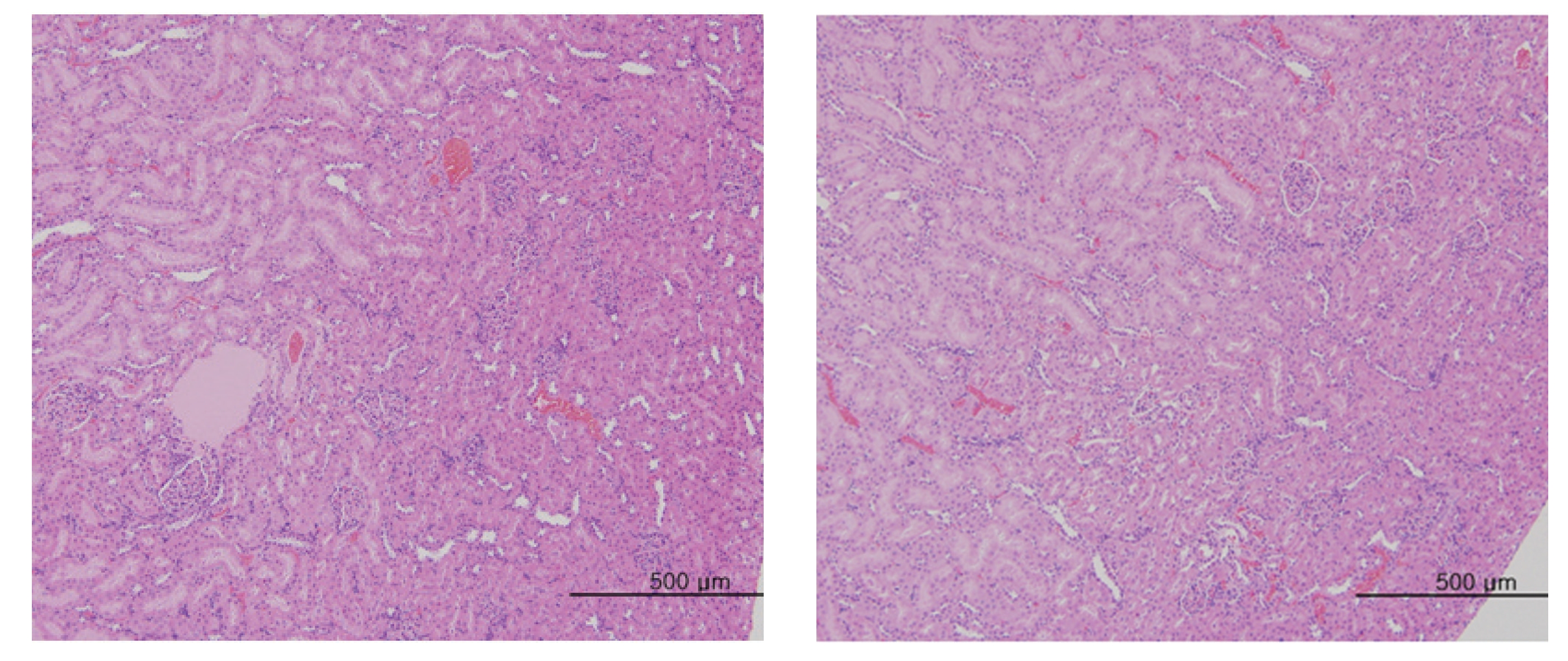
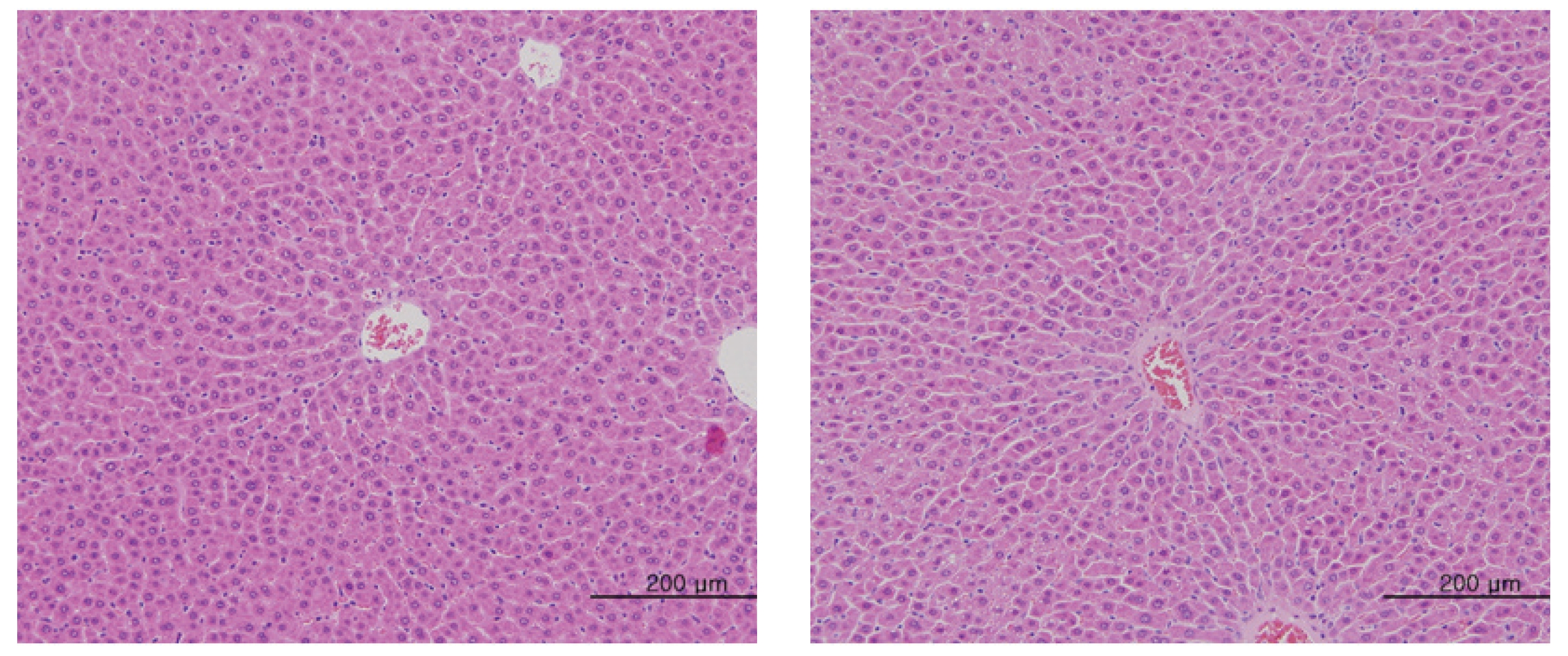
No abnormal macroscopic appearances were observed on necropsy in either the control or the experimental groups. All experimental groups showed no abnormal signs caused by intravenous injection of MGP. In the control and the high-dosage groups, some changes were observed in the kidney, lung, liver and spleen, but those changes seemed to have occurred naturally or accidentally (Fig 3-6).
Blood vessels are consist of veins and arteries [10, 11]. MGP is administered intravenously, and intravenous injection is enlisted as a legal manipulation in Korean classification procedures in medicine and resource. For this reason, intravenous injection of MGP has been studied [1].
In a randomized controlled trial of MGP, no significant changes in blood pressure, hematology, biochemistry and urine analysis were reported [12]. However, immune protein, which acts against microbes and pathogenic organisms, vitamin D binding protein, which protects the lung at the time of inflammatory response, ras-related protein Ral-A, which controls phospholipid metabolism, cytoskeletal formation, and membrane traffic, and transferrin which balances the iron level in the body were reported to be increased significantly, after the administration of MGP, but antitrypsin, which is secreted with inflammatory responses in the lungs, were reduced [13, 14]. Also no effects on heart rate variability [15] and pulse-wave factors were revealed in middle-aged women [16].
There are many types of flavonoid compounds, such as quercetin, hesperidin and anthocyacanidins in MGP, and these constituents were reported to have actions that improved liver functions [17, 18], and that produced anti-oxidant [19-23], anti-cancer [7, 24-30], anti-hypertensive [31], anti-diabetic [32], anti-obesity [33] and anti-depressant effects [34]. Thus, MGP has been used to treat progressive disorders such as cancer [35-40] and amyotropic lateral sclerosis [41].
The present study was conducted to evaluate the safety of MGP, and the results of this study showed no mortalities and no significant changes in SD rats caused by intravenous injection of MGP. These results correspond with those of an earlier study which reported that MGP caused negligible toxicity [24]. Although the safety of MGP has been demonstrated through a single-dose toxicity test, still further study, like a 4-week repeated toxicity test, is needed. Based on the results of this study, we can conclude with certainly that the lethal dose of MGP is higher than 20 mL/kg in SD rats and that intravenous injection of MGP in SD rats is a safe treatment considering that the usual clinical dose of MGP is about 0.3 mL/kg.
The field of traditional korean medicine (TKM) has a long waited technology that uses the vessel as a treatment medium, as the vessel provides the quickest path of drug ab-sorption. A limitation on treating patients is the difficulty of oral administration of medicines, but pharmacopuncture, especially MGP has opened the door for immediate treatment benefits. As the result of this study, we can conclude with certainly that intravenous injection of MGP is safe.