This study was performed to analyze the toxicity and to find the lethal dose of the test substance Water-soluble Carthami-flos pharmacopuncture (WCF) when used as a single intravenous-dose in 6-week-old, male and female Sprague-Dawley rats.
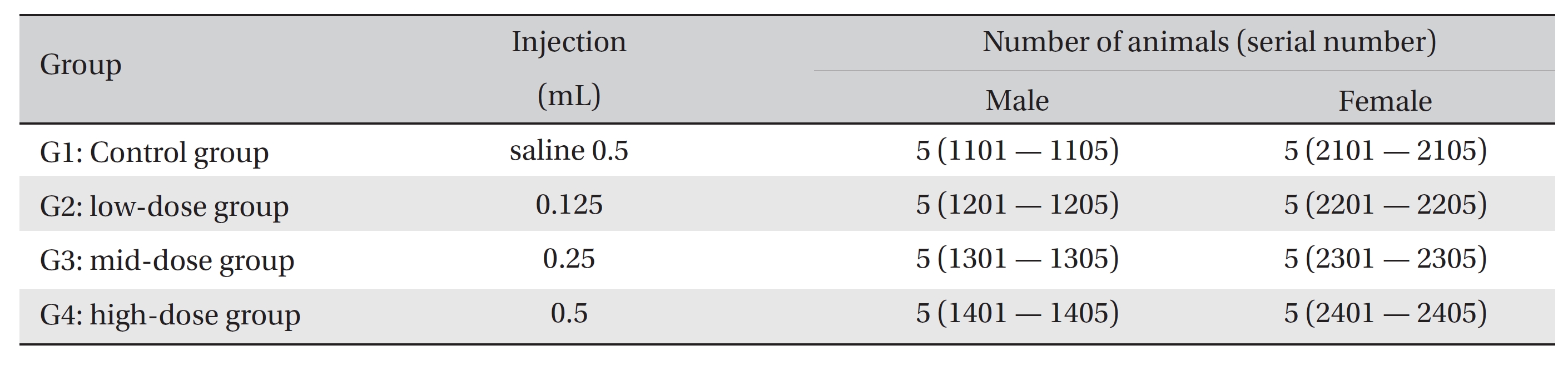
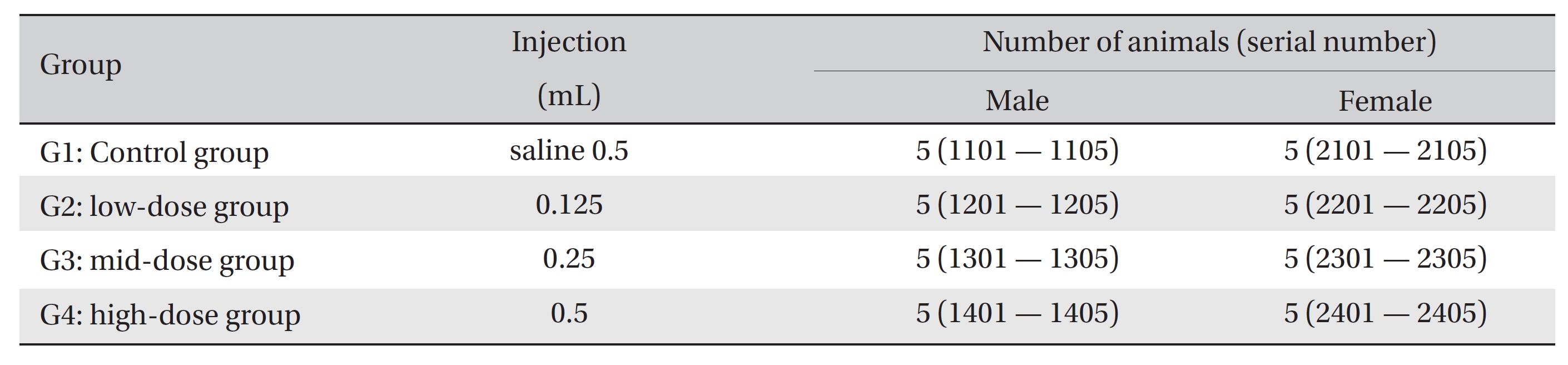
The experiment was conducted at Biotoxtech according to Good Laboratory Practices. 20 female and 20 male Spague-Dawley rats were divided into 4 groups of 5 female and 5 male animals per group. The rats in the three experimental groups received single intravenous injections with 0.125-mL, 0.25-mL and 0.5-mL/animal doses of WCF, Groups 2, 3, and 4, respectively, and the control group, Group 1, received a single intravenous injection with a 0.5-mL dose of normal saline. Clinical signs were observed and body weight measurements were carried out for 14 days following the injections. At the end of the observation period, hematology, clinical chemistry, histopathological tests and necropsy were performed on the injected parts.
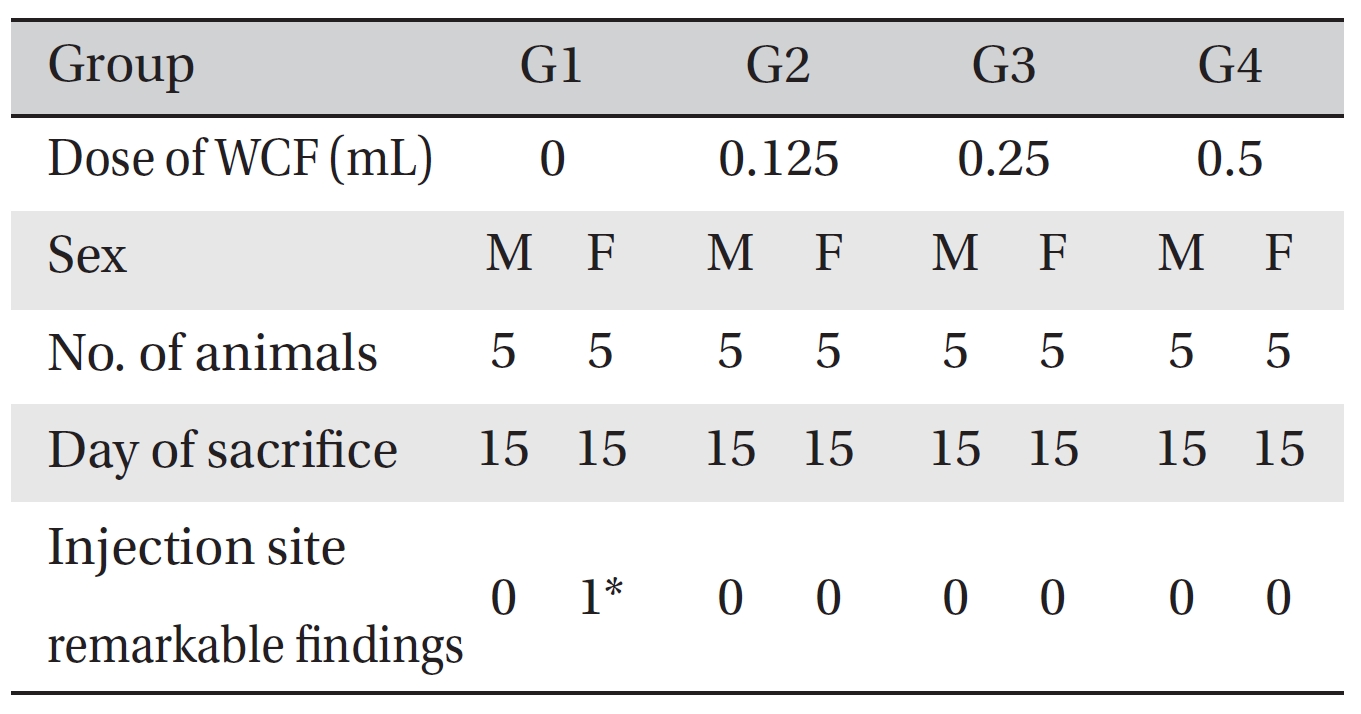
No deaths occurred in any of the groups. Also, no significant changes in body weight, hematological parameters or clinical chemistry test results between the control group and the experimental groups were observed. Visual inspection after necropsy showed no abnormalities. Histopathological tests on the injected parts showed no significant differences, except for Group 1 females; however, the result was spontaneous generation and had no toxicological meaning because it was not dose-dependent. Therefore, this study showed that WCF had no effect on the injected parts in terms of clinical signs, body weight, hematology, clinical chemistry, and necropsy.
As a result of single intravenous-dose tests of the test substance WCF in 4 groups of rats, the lethal dose for both males and females exceeded 0.5 mL/animal. Therefore, WCF is a relatively safe pharmacopuncture that can be used for treatment, but further studies should be performed.
Pharmacopuncture is a new form of acupuncture treatment combining acupuncture and herbal medicine. With acupuncture being based on the meridian theory and herbal medicine being based on Qi and flavor theory, pharmacopuncture utilizes both the meridian and the Qi and flavor theories. Pharmacopuncture is a unique treatment of traditional Korean medicine and uses needling and herbal medicine based on Qi and flavor theories and acupoint selection based on meridian theories [1].
Many studies have demonstrated the effects of CF. Yoon
Water soluble Carthami-flos herbal acupuncture (WCF) is prepared by homogenizing CF, water for injection (WFI), and an emulsifier [6]. WCF is widely used nowadays, and Lee
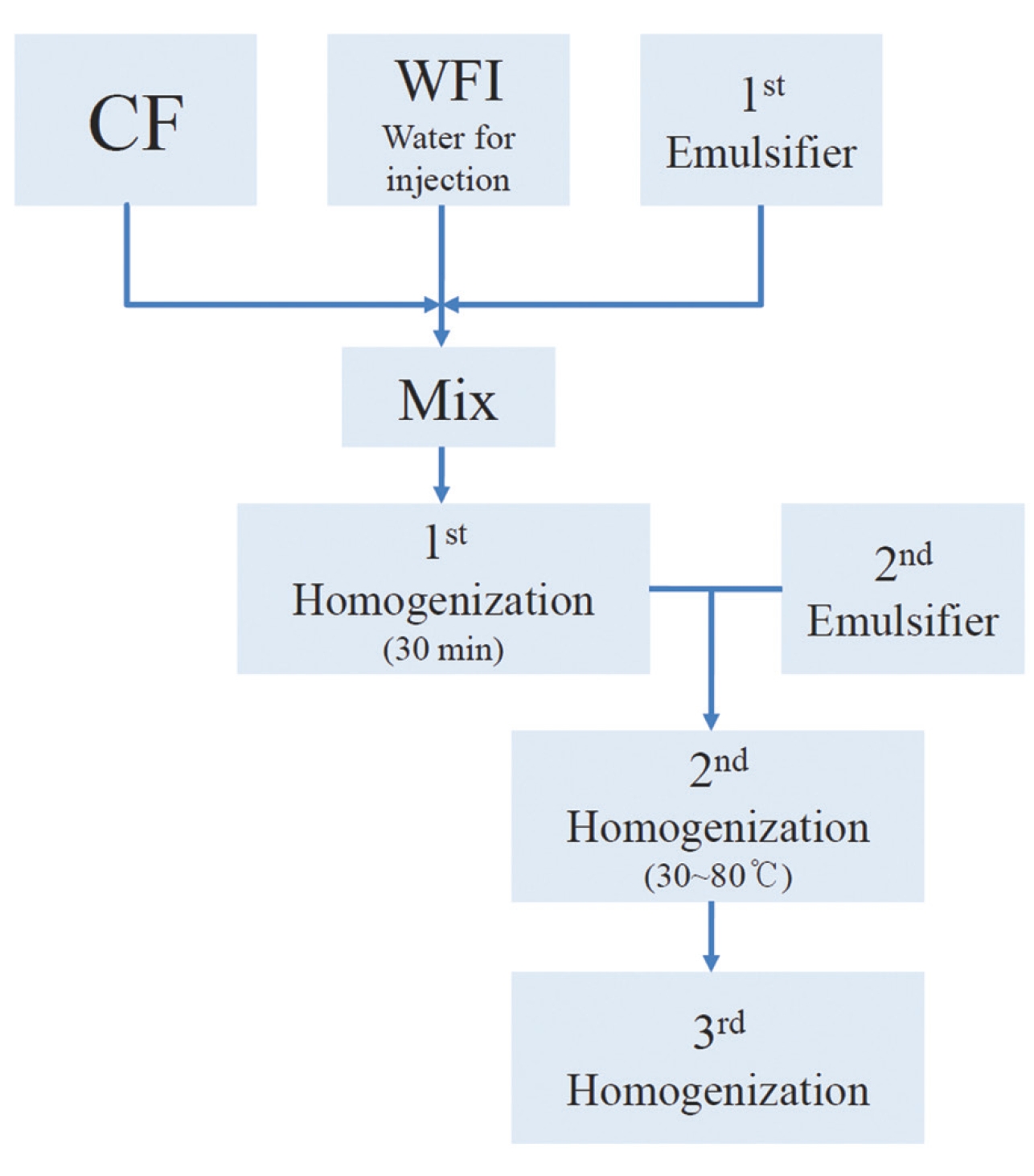
The WCF was provided by the Korean Pharmacopuncture Institute (KPI). WCF was developed both to increase the treatment effects by increasing the absorptivity and to decrease the side effects by reducing the residue of existing CF. WCF is prepared in three steps: In step 1, CF, WFI, and a primary emulsifier are mixed together and homogenized for 30 minutes by using a high-speed homogenizer. The emulsifier is added in step 2 and homogenized at 30 — 80℃. In step 3, the temperature is reduced, and the mixture is homogenized to yield WCF [6] (Fig 1).
24 male and 24 female SD rats were purchased from ORIENTBIO Inc. The 24 male and the 24 female rats were 5 weeks old with body weights of 117.5 — 129.3 g, and 104.2 — 128.2 g, respectively. The laboratory conditions were as follows a temperature of 21.0 — 22.8℃, a relative humidity of 42.9% — 59.3%, 10 - 15 ventilations/hour, a lighting cycle of 12 hours/day, and an illuminance of 150 — 300 Lux.The rats were supplied with food (Teklad Certified Irradiated Global 18% Protein Rodent Diet 2918 C) and clean water (tap water of Cheongju filtered with a water sterilizer and irradiated with ultraviolet rays). After 7 days of acclimatization, the weights of the male rats were 184.1 — 203.8 g, and those of the female rats were 144.8 — 169.9 g. The 6-week-old SD rats were grouped by using the criteria of their weights being close to the mean weight. A total of 20 male rats and 20 female rats were distributed into 4 groups with 5 mice of each sex per group. The remaining animals were excluded from the test.
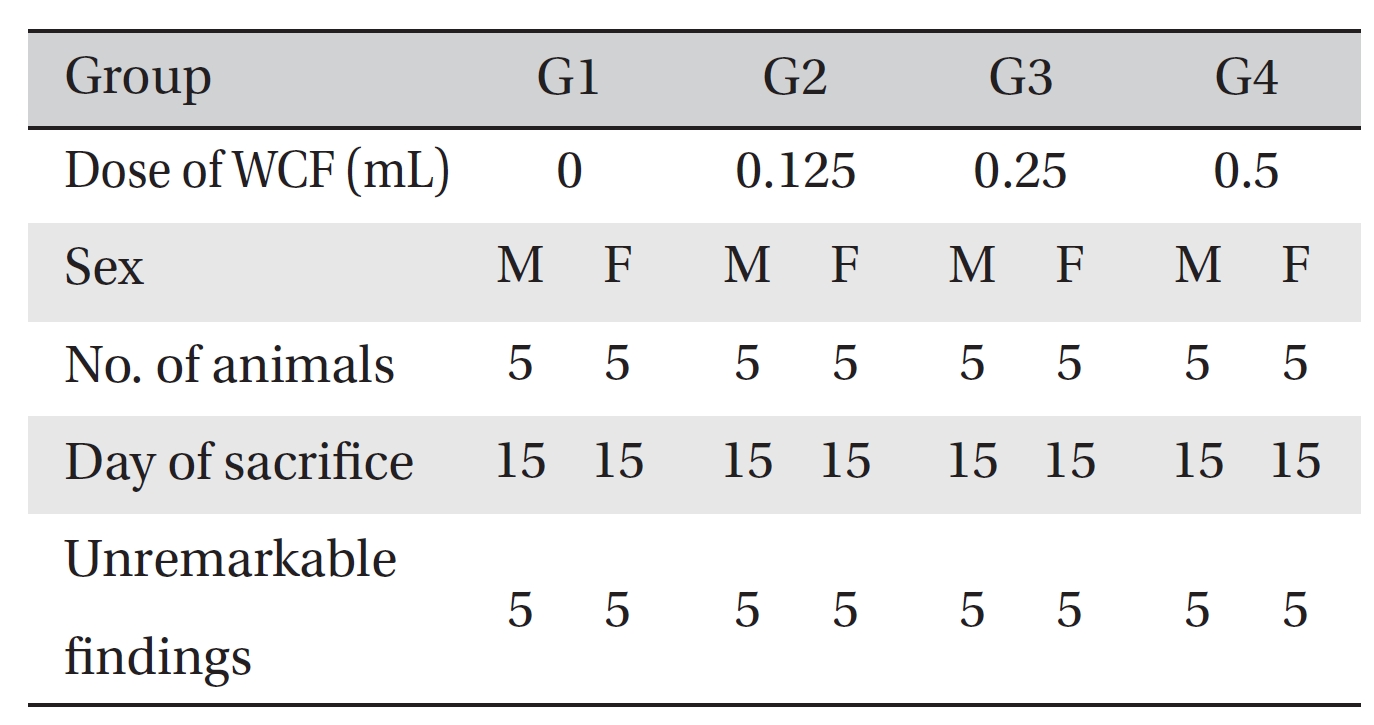
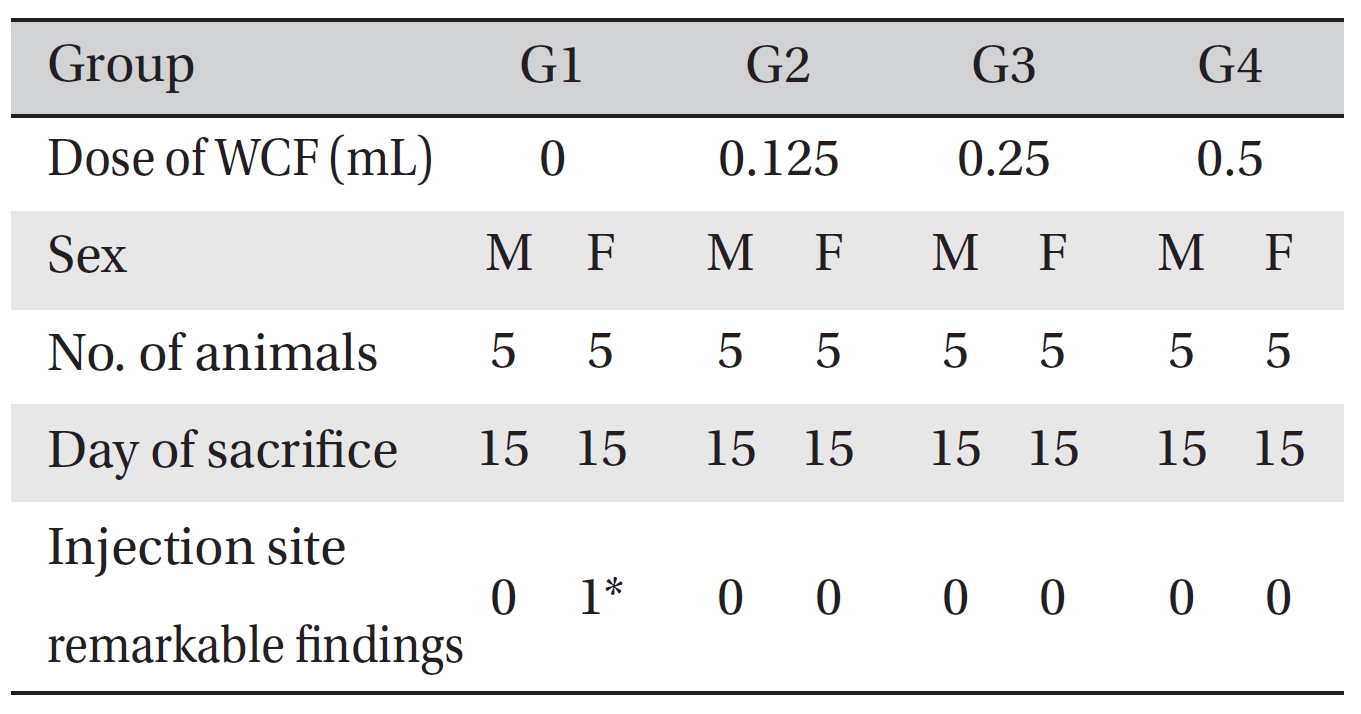
No deaths were observed as a result of single intravenous- dose in the male or the female rats during preliminary test (Biotoxtech Study No.: B13481P). Based on those results, 0.5 mL/animal was selected as the high dose (Group 4: G4), and 0.25 and 0.125 mL/animal as the middose and the low dose (Group 3: G3 and Group 2: G2, respectively). Normal saline solution, 0.5 mL/animal, was used for the control group (Group 1: G1) (Table 1).
[Table. 1] Grouping of the rats
Grouping of the rats
CF was prepared in a sterile laboratory at the KPI. After the seeds had been cleaned, ground and peeled, they were screw pressed on a prepared mat. Rough unwanted residue was removed, and natural precipitation was induced. The extract from a 3-step filter was packaged with nitrogen [7].
Injection was performed using a disposable syringe (1 mL, 26 G). In G2, 0.125 mL of WCF was injected through the tail vein. In G3, 0.25 mL of WCF was injected through the tail vein as in G2. In G4 and G1, 0.5 mL of WCF and normal saline was injected through the tail vein, respectively. An intravenous dose was selected because WCF should be clinically applied to veins and muscles. This study was conducted under the approval of the Institutional Animal Ethics Committee.
Body weights, hematology, and clinical chemistry test results were tested using SAS (version 9.3, SAS Institute Inc., U.S.A). The Bartlett test was performed for homoscedasticity (significance level: 0.05). In the case of homoscedasticity, a one-way analysis of variance (ANOVA) was performed to yield significance (significance level: 0.05), and multiple testing of Dunnett’s t-test was carried out (significance level: 0.05 on both sides and 0.01). For heteroscedasticity, significance (significance level: 0.05) was checked by using the Kruskal-Wallis test.
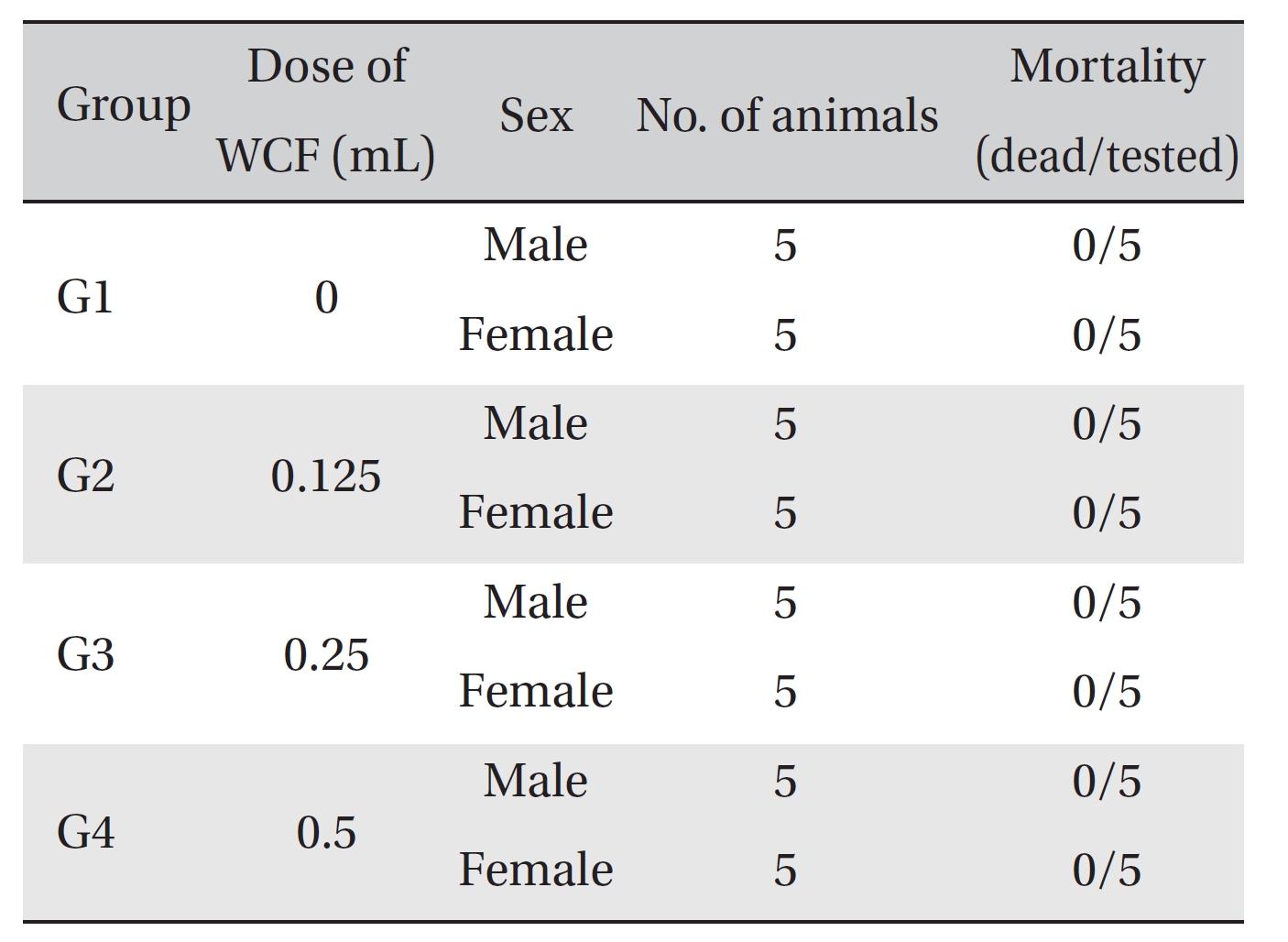
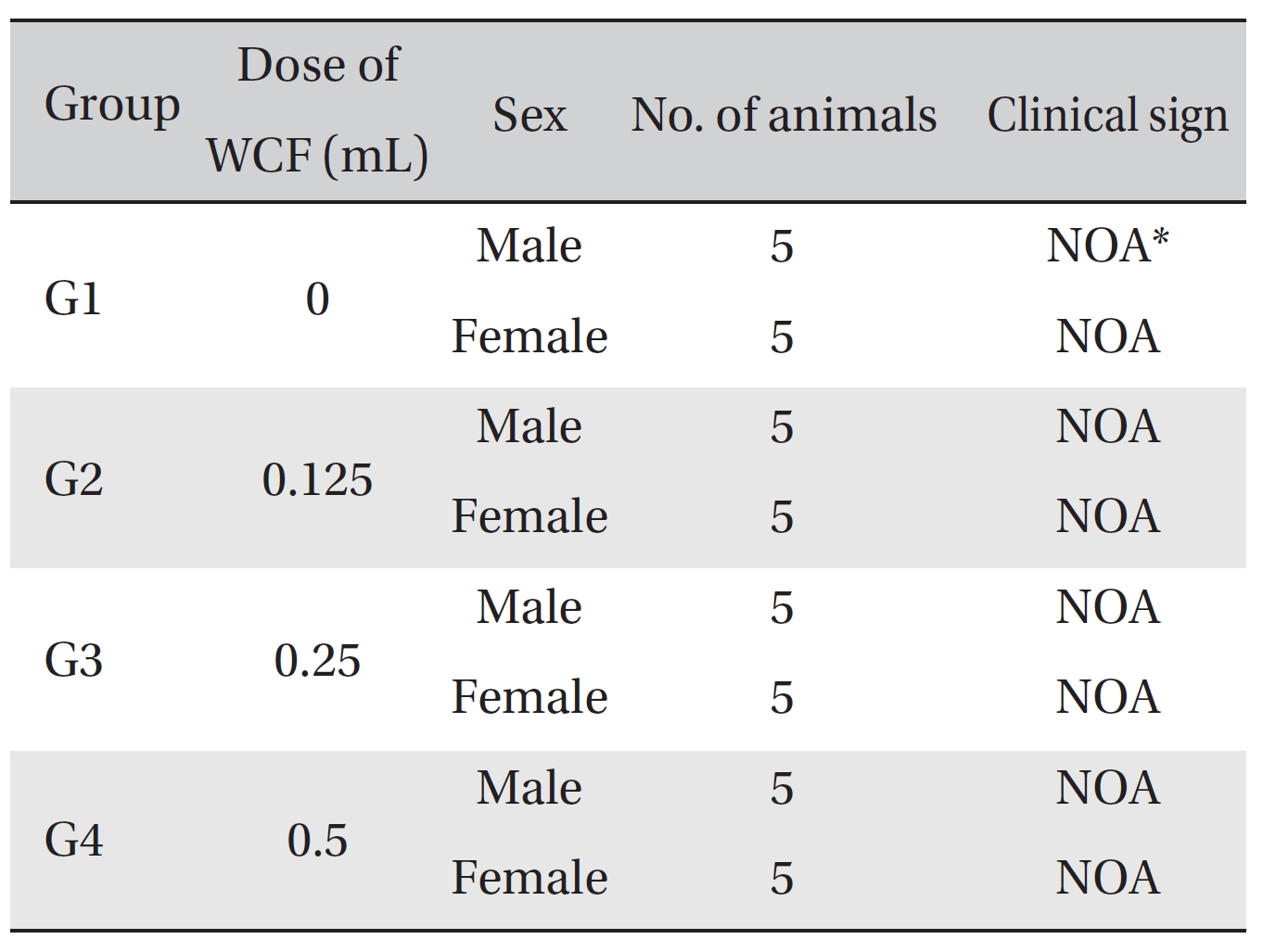
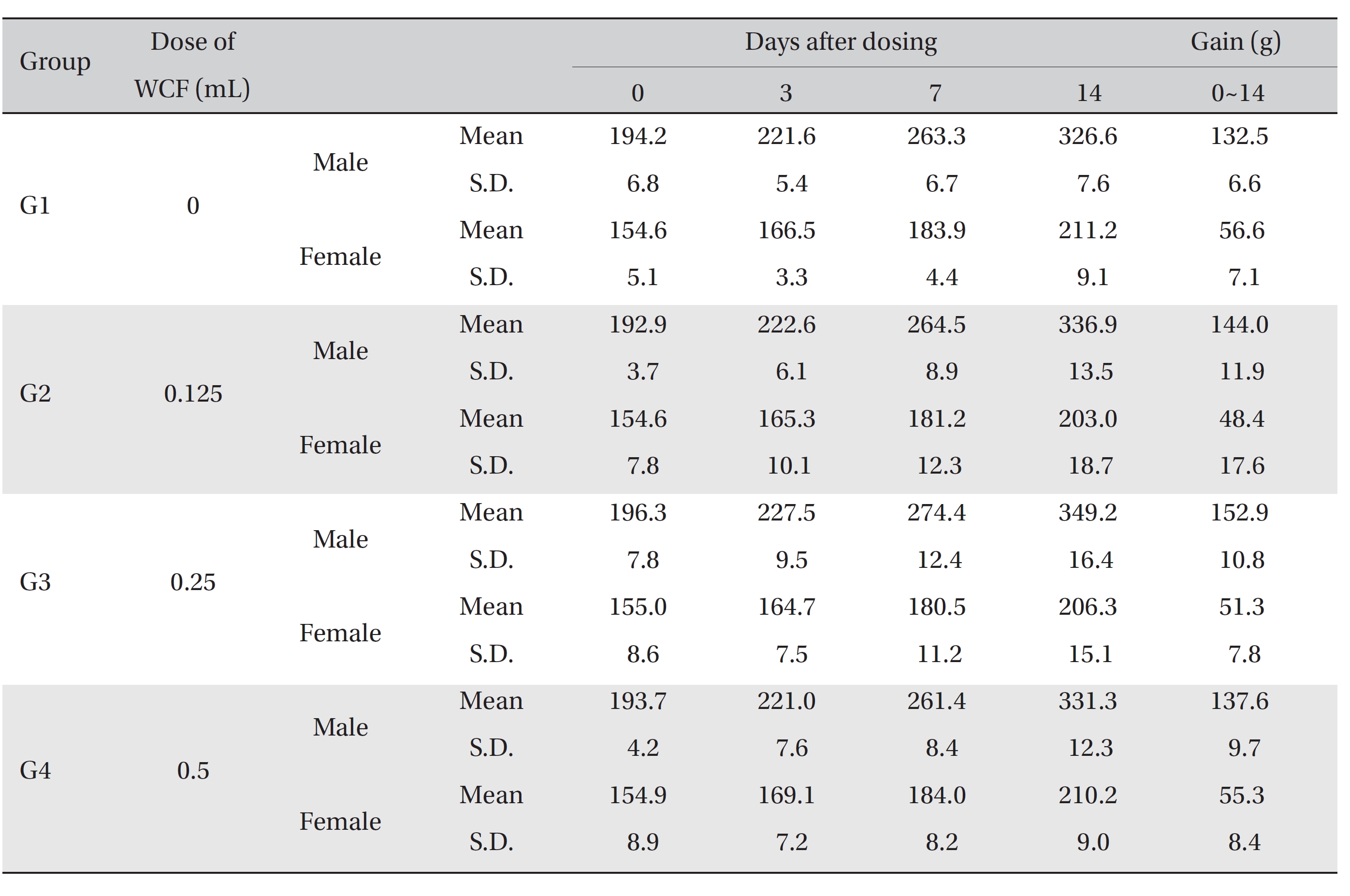
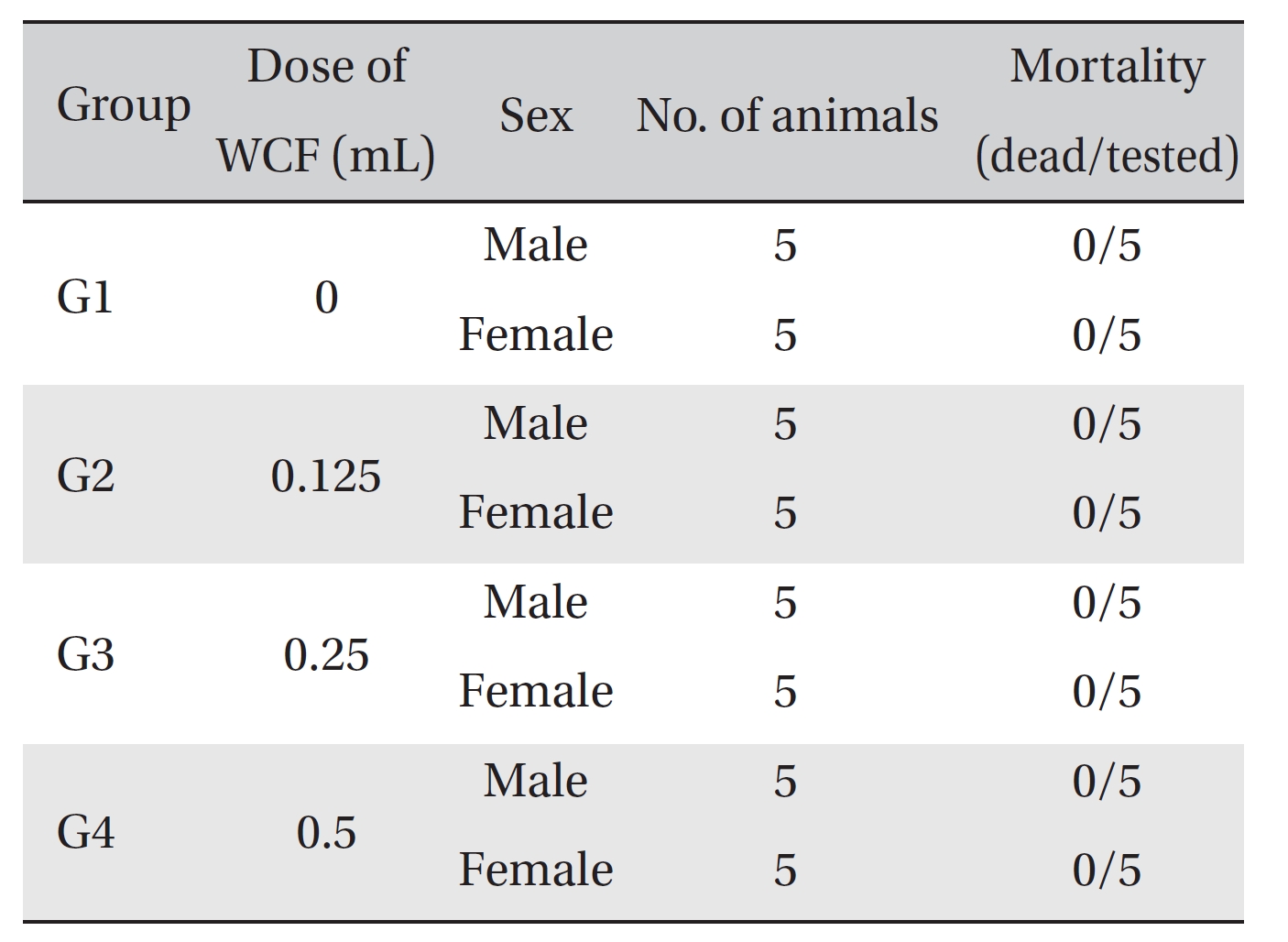
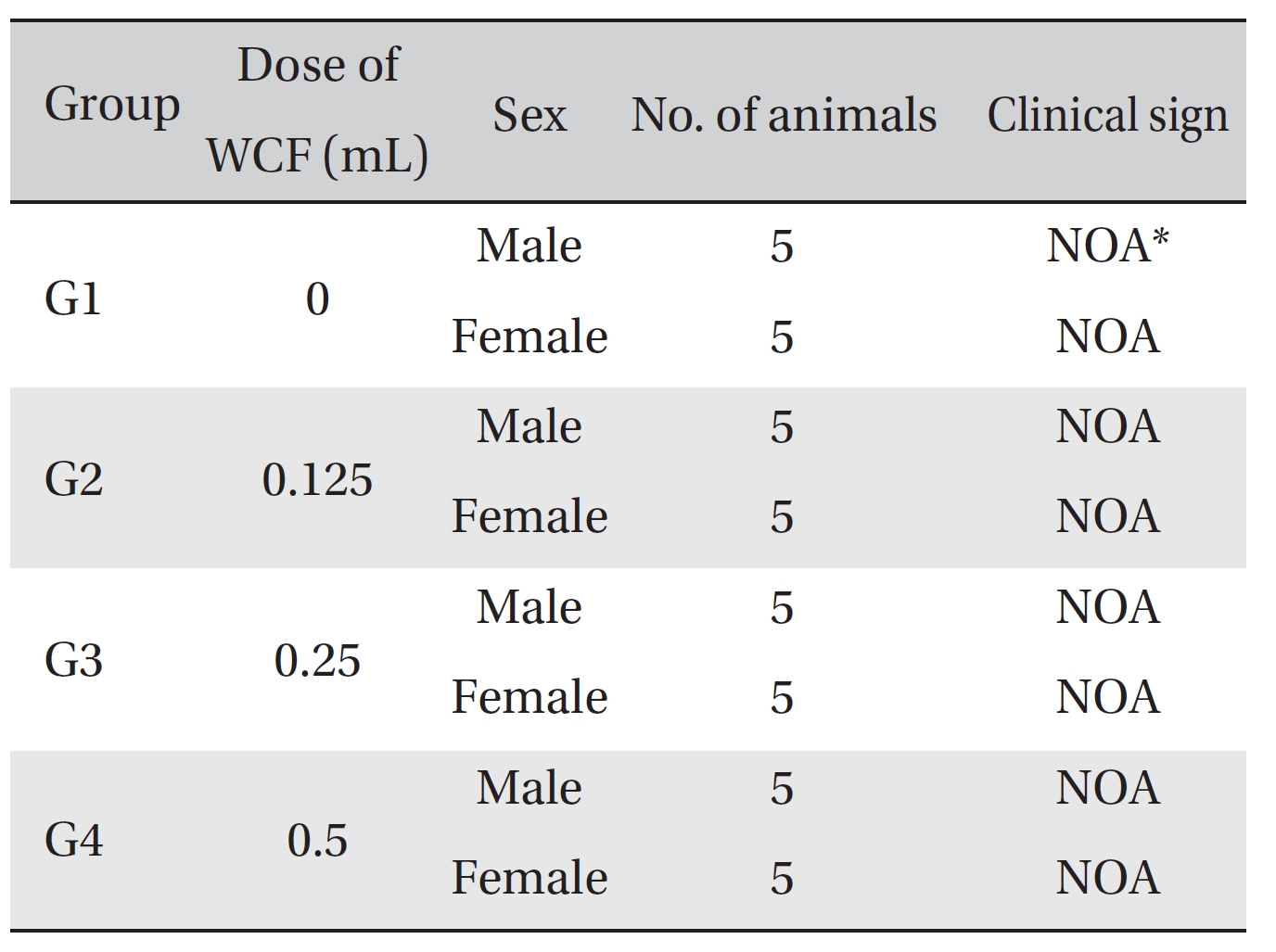
No mortalities occurred in any group (Table 2) Therefore, computation of the LD50 was impossible; however, a single intravenous dose of WCF at 0.5 mL/animal caused no mortalities. In addition, on the day of injection (day 0), clinical symptoms (type of toxicity symptom, time of expression, time of recovery, etc.) and fatalities were observed at 30 minutes, 1, 2, 4 and 6 hours after injection. From day 1 to day 14, clinical symptoms were observed once a day. As a result of observations until day 14 after injection of WCF, no abnormalities were found in any of the experimental rats (Table 3) Body weight was measured on the day of injection (before injection) and on days 3, 7 and 14. All groups showed continued increase in body weight after selection of the experimental groups. No significant differences existed among the experimental groups (Table 4).
Mortality
[Table. 3] Clinical signs from day 0 to day 14
Clinical signs from day 0 to day 14
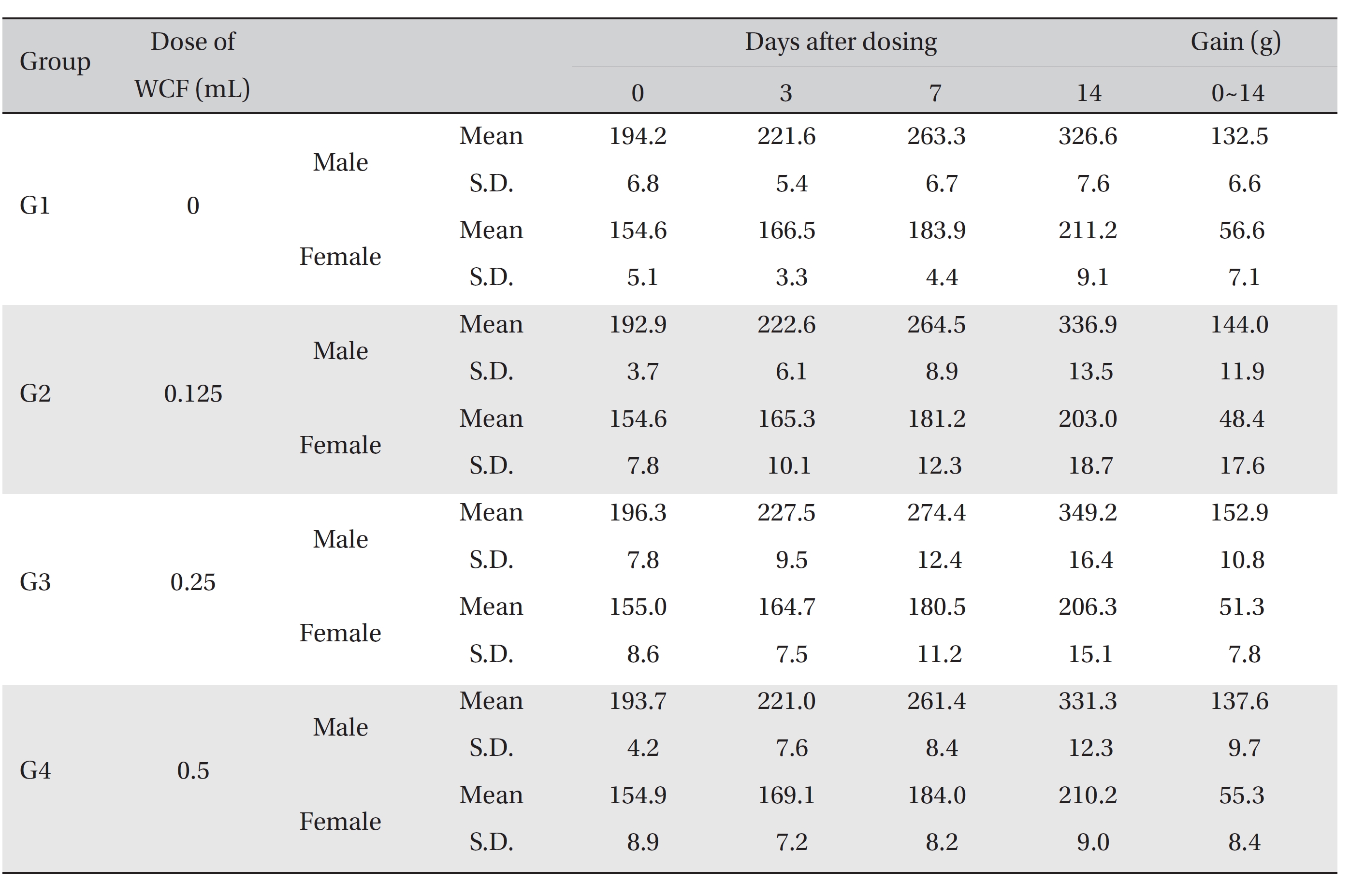
Mean body weights
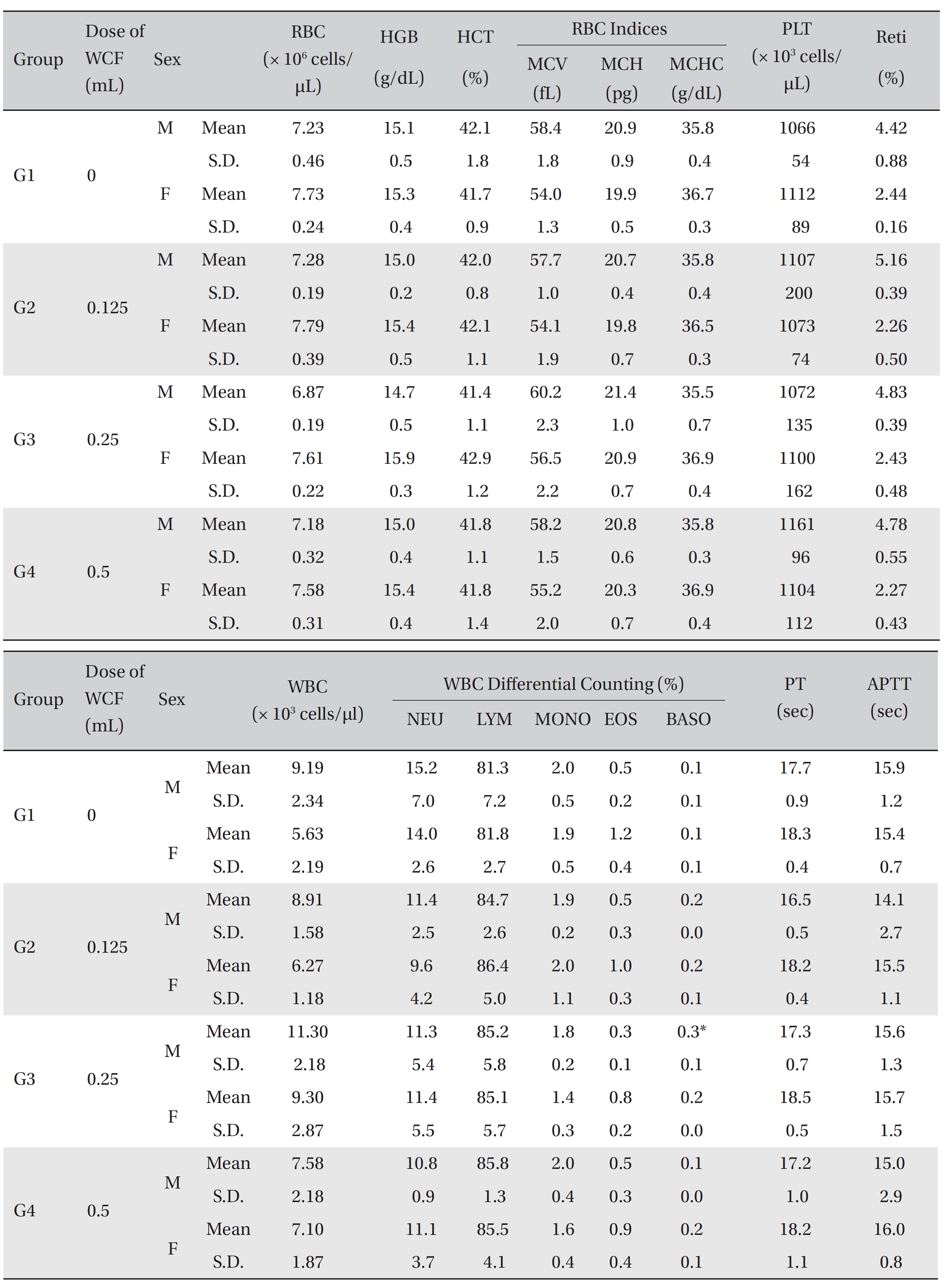
[Table. 5] Mean hematology parameters
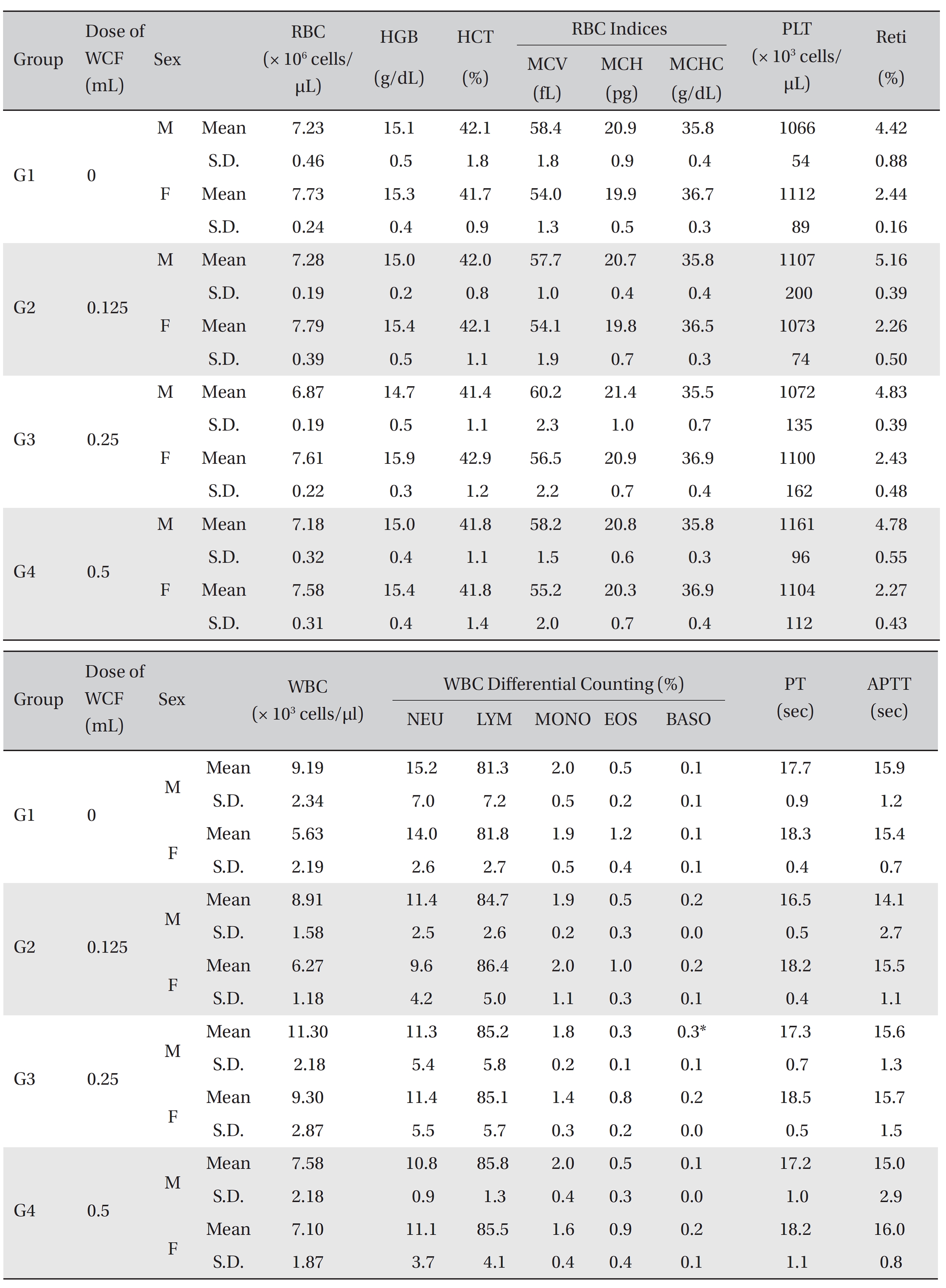
Mean hematology parameters
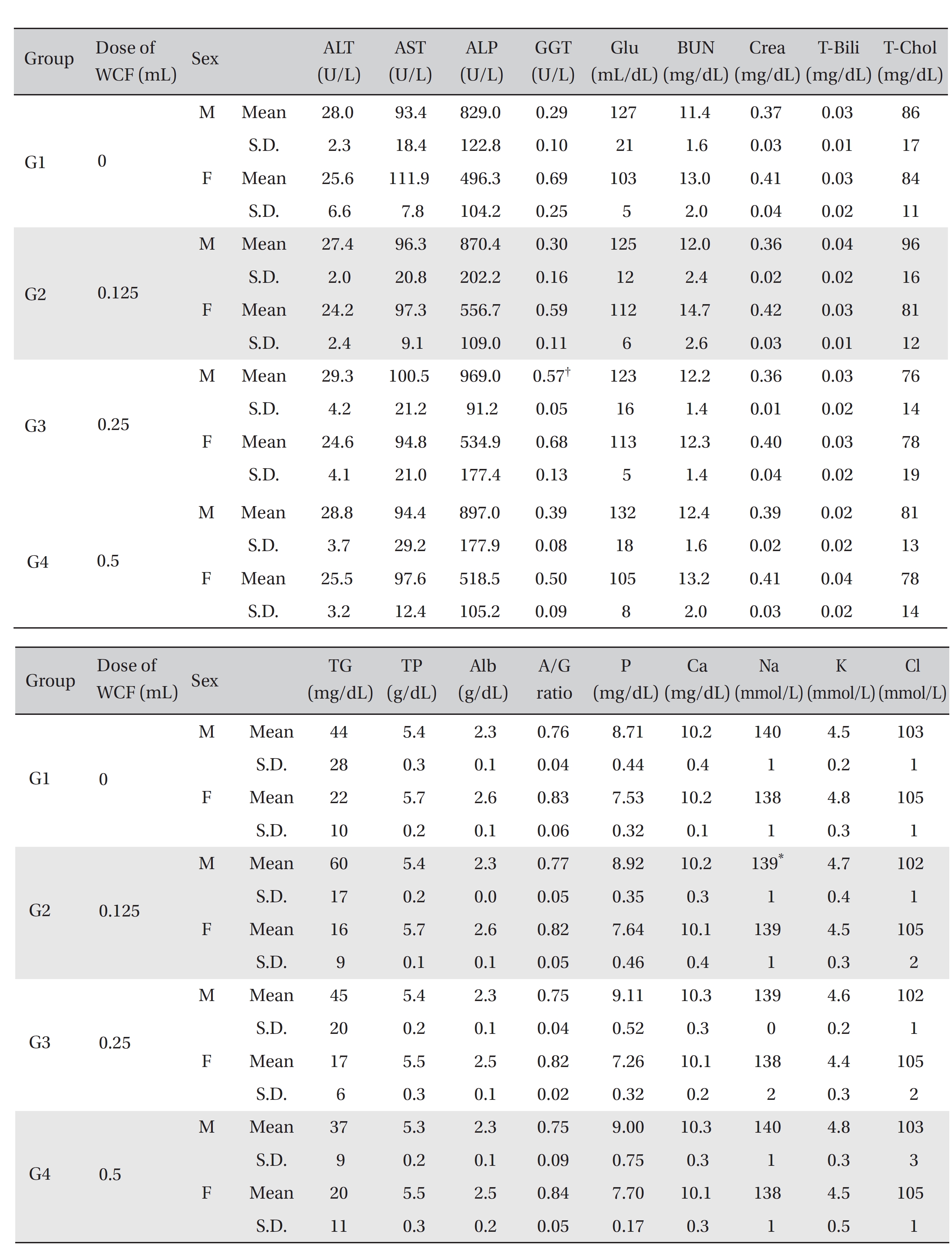
[Table. 6] Mean clinical chemistry
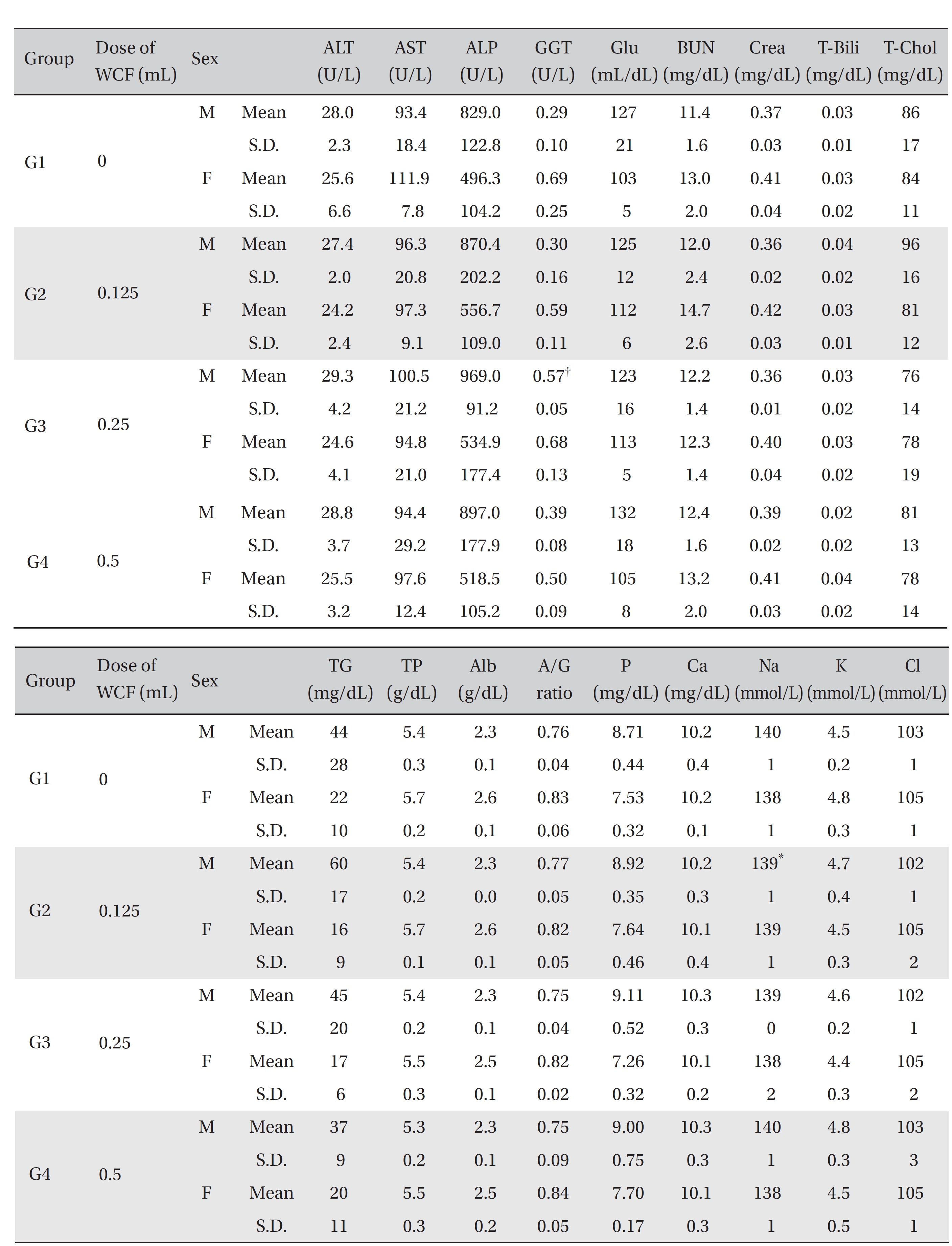
Mean clinical chemistry
All rats had been fasting for more than 18 hours before necropsy. Blood was collected from the abdominal aorta on day 15 after applying anesthesia with isoflurane. The hematology test was done by using a blood corpuscle analyzer (ADVIA 120, SIEMENS, Germany) after placing about 1 mL of the collected blood in a tube containing ethylenediaminetetraacetic acid (EDTA). Erythrocyte count (RBC), hemoglobin (HBG), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH),mean corpuscular hemoglobin concentration (MCHC), platelet (PLT), leucocyte count (WBC), WBC differential count (neutrophils, lymphocytes, monocytes, eosinophils, basophils) and Reti (reticulocytes) were measured. For the coagulation test, about 2 mL of the collected blood was placed in a tube containing 3.2% sodium citrate and was centrifuged for 10 minutes at 3,000 rpm before collecting the blood plasma. Measurement was done using a coagulation time analyzer (Coapresta 2000, SEKISUI, Japan). The prothrombin time (PT) and the activated partial thromboplastin time (APTT) were measured. The hematology test showed no significant differences between the normal saline group and any of the WCF groups, except that the BASO of G3 males showed statistically significant changes with P < 0.01; however, the result had no toxicological meaning because it was not dose-dependent (Table 5).
For the clinical chemistry test, the unused blood remaining after the hematology test was centrifuged for 10 minutes at 3,000 rpm before blood serum was collected. A clinical chemistry analyzer (7180, HITACHI, Japan) and an electrolyte analyzer (AVL9181, Roche, Germany) were used to measure alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT), blood urea nitrogen (BUN), creatinine (Crea), total bilirubin (T-Bili), total protein (TP), albumin (Alb), A/G ratio, total cholesterol (T-chol), triglycerides (TG), phosphorus (P), glucose (Glu), calcium (Ca), chloride (Cl), sodium (Na) and potassium (K). The clinical chemistry test showed no changes caused by the test substance in animals of either sex in any group, except that the Na of G2 males and the GGT of G3 males showed statistically significant changes with P < 0.05, P < 0.01, respectively; however, the result had no toxicological meaning because it was not dose-dependent (Table 6).
Visual inspection of all body organs and tissues was performed on all animals after necropsy. Body organs and tissues were extracted and fixed in 10% neutral buffered formalin, and histopathological observations were carried out. Visual inspection after necropsy showed no visual abnormalities in any of the male or the female animals in any experimental group (Table 7) In addition, histopathological tests on the injected parts showed no effect due to the test substance in any animals, except G1 females; however, the result was due to spontaneous generation and had no toxicological meaning because it was not dose-dependent (Table 8).
Necropsy findings
[Table. 8] Histopathological findings
Histopathological findings
Pharmacopuncture can produce the effects of both acupuncture and herbal medicine. Also, a synergistic effect would be expected because pharmacopuncture does not pass through the digestive system [8]. The treatment area of pharmacopuncture is the common acupoints, tender points, or responsive points that are relevant to other disorders. In the procedure, two different types of pharmacopuncture are used: aromatic and oil-based pharmacopunctures. Oil-based pharmacopunture is used for consumptive diseases or degenerative diseases, and the extracts of
Oil-based pharmacopuncture is used for kinetic disorders caused by excessive usage causing a deficiency of body lubrication, for consumptive disorder induced by lopsided fire and dryness from improper ascending water and descending fire, and for disorders in the elderly such as arthritis, arteriosclerosis, and hypertension. CC, JSD, and CF, where oil is the main component, are commonly used. CF is used to treat vertebral disorders (lumbar pain in the elderly, coccyalgia, and fractures), joint disorders (degenerative arthritis, sprains, and tennis elbow), soft tissue damage (frozen shoulder, plantar fasciitis, and sequela of sprain), contusions, menstrual irregularities, deficient- type constipation, and muscle induration [9]. CF can be used not only for pain relief [10] but also for a significant increase in regional cerebral blood flow [11]. Oh
Oil-based pharmacopunctures such as CC, JSD, and CF, which are made with alcohol extraction or compression, require more attention than
WCF was newly developed to provide strong effects and to reduce side effects. WCF is prepared by homogenizing a mixture of CF, WFI, and an emulsifier and increases the treatment efficacy while maintaining the characteristics of existing CF [6]. In this study, an intravenous-dose toxicity test of WCF was performed to evaluate the stability of WCF, which had been manufactured by the KPI according to Good Laboratory Practices. No mortalities occurred in any group. Also, no abnormalities in the clinical symptoms were observed in any of the four groups. No significant body weight differences were observed in any group. Hematologically, a change in the BASO of the G3 male group was observed, but that change had no clinical or toxicological meaning. The clinical chemistry test showed changes in the Na of the G2 male group and the GGT of the G3 male group, but those changes had no clinical or toxicological meaning. A clinical chemistry study with a greater number of subjects and for a longer period is deemed necessary to examine the long-term and/or the high-dose effects of WCF. Visual inspection after necropsy showed no visual abnormalities in the male and the female animals in any experimental group. Histopathological tests on the injected parts showed a cell infiltration in the G1 female group; however, the result was due to spontaneous generation and had no toxicological meaning.
WCF is expected to be widely used. Toxicity, dose, and side effects must be studied because pharmacopuncture inserts foreign substances into the human body. In this study, single intravenous-dose toxicity tests were performed on rats to verify the safety of WCF and to identify its side effects. The result of single intravenous-doses of WCF in the 4 groups of rats showed that the approximate lethal dose for both male and female rats exceeded 0.5 mL. Therefore, WCF is a relatively safe pharmacopuncture that can be used for treatment, but further studies should be performed in the future. Also, the toxicity and the safety of WCF for use on humans must be investigated.
In this study, intravenous injection of WCF did not cause any problems. As none of the test subjects was affected by injections of up to 0.5 mL of WCF, levels over 0.5 mL may have a detrimental effect. If the exact lethal dose is to be established, further studies are needed. Finally, the results clearly show that WCF dosing up to 0.5 mL is safe.