Neurofibromatosis type 1 (NF1), known as Von Recklinghausen disease, is one of the most common gene disorders to human. It has an incidence of 1 per 3,500 - 4,000. Pharmacopuncture is a type of neo-acupuncture that treats diseases by injecting herbal fluids at acupoints to gain the simultaneous effects of acupuncture and herbal medicine [1].
The previous experimental study, however, only proved the anti-obesity effect by administrating dried samples of
The
Forty six-week-old Sprague-Dawley (SD) rats were used in this experiment: 20 male rats (body weights: 193.9 ─ 210.5 g) and 20 female rats (body weights: 144.3 ─ 178.0 g). Visual inspections and measurements of body weight by using electronic scales (CP3202S, Sartorius, Germany) were conducted on all animals when they were brought into the experiment. The general symptoms were observed once a day prior to the start of the experiments, with males and females within each groups being weighed and examined for general symptoms and body-weight changes on the last day of acclimatization. No abnormalities were found.
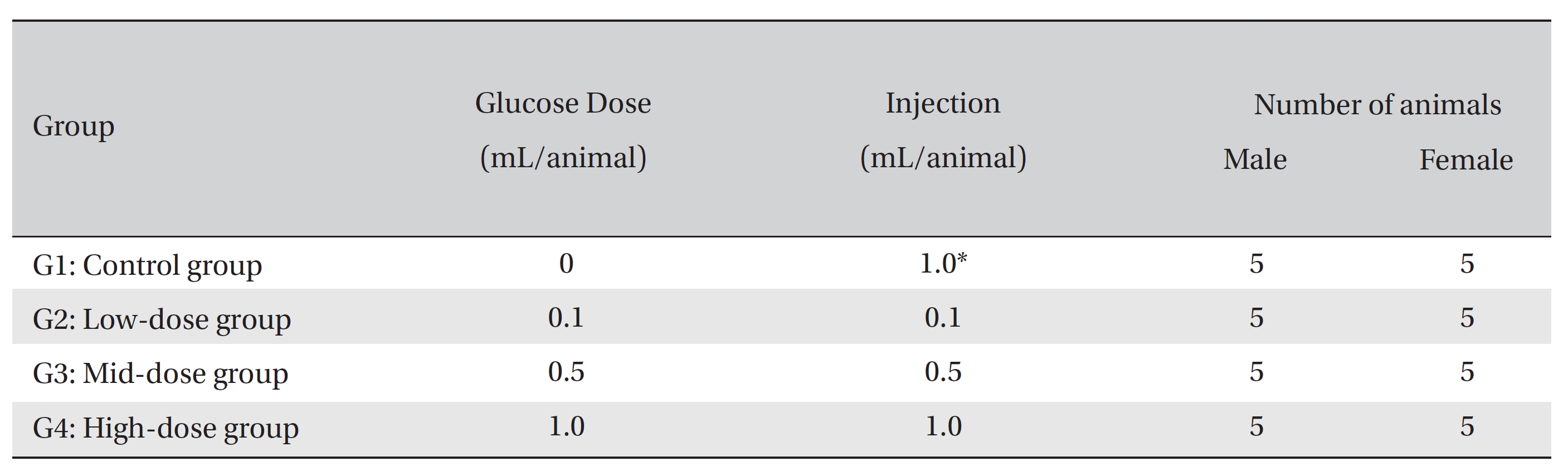
Group separation was conducted on the last day of acclimation. All animals were randomly distributed into four different groups with five individuals of each sex per group. The four different groups were labeled as follows: Group 1 (G1, control group): normal saline solution, Group 2 (G2): low-dose group, Group 3 (G3): mid-dose group, and Group 4 (G4): high-dose group (Table 1)
In a pilot test (Biotoxtech Study No.: B12880P), 1.0 mL/ animal was administered through an intravenous route to one male and one female rat, which resulted in no deaths. From this result, the doses for
This experiment was conducted at Biotoxtech, an authorized institution for non-clinical studies, under the regulation of Good Laboratory Practice (GLP) of KFDA Notification No. 2012-61 (Guidelines for non-clinical laboratory studies, Aug 24, 2012) [6].
The general symptoms and mortality were observed after 30 minutes, 1, 2, 4, and 6 hours on the day of injection (day 0). From the next day to the 14th day after the injection, the general symptoms were examined once a day.
[Table. 1] Compositions of the experimental groups
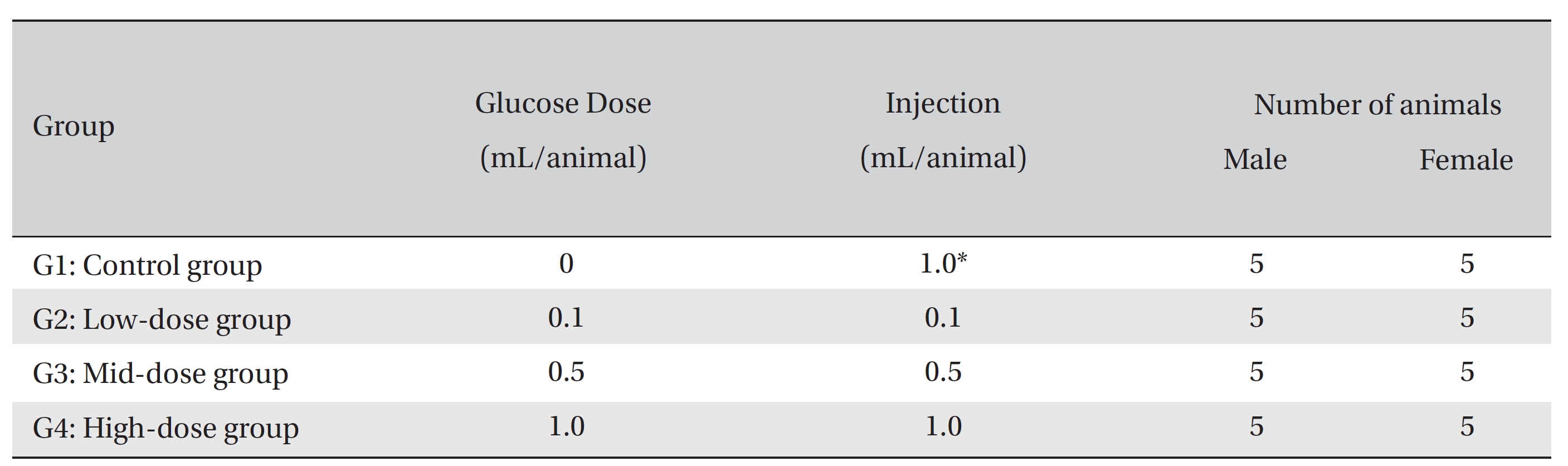
Compositions of the experimental groups
The body weights were measured on the day of the injection and on the 3rd, 7th, and 14th day after the injection.
All animals were fasted during 18 hours before autopsy, the 15th day after the injection. They were then anesthetized with isoflurane, after which blood was collected from the abdominal aorta. For the hematological test, about 1 mL of the collected blood was placed in an ethylene-diamine-tetra-acetic acid tube and was analyzed with a hematology analyzer (ADVIA® 120, Siemens, Germany). For the coagulation test, about 2 mL of the collected blood was placed in a tube with 3.2% sodium citrate and centrifuged at 3,000 rpm for 10 minutes. Blood plasma was then collected. Different laboratory tests were conducted using a coagulation time analyzer (Coapresta® 2000, Sekisui, Japan).
For the biochemical test, the blood remaining after carrying out the hematological tests was centrifuged at 3,000 rpm for 10 minutes, and the serum was collected. Tests were done using a biochemistry analyzer (7180, Hitachi, Japan) and an electrolyte analyzer (AVL9181, Roche, Germany).
During the necropsy performed on all the animals, organs and tissues from the entire body were checked thoroughly by visual inspection.
Tissues at the injection sites for all the animals were sampled and fixed in 10% neutral buffered formalin. Routine histological methods, such as trimming, dehydration, and paraffin embedding, were conducted on the fixed organs and tissues. These were then sliced using a microtome and stained with hematoxylin & eosin (H&E).
All the results obtained were analyzed by using STATA/SE 9.2 for Windows (StataCorp LP, College Station, TX, USA). The equal variance was tested using Bartlett’s test. If the sample variances were equal, significant results were obtained using the one-way analysis of variance. Dunnett’s multiple range
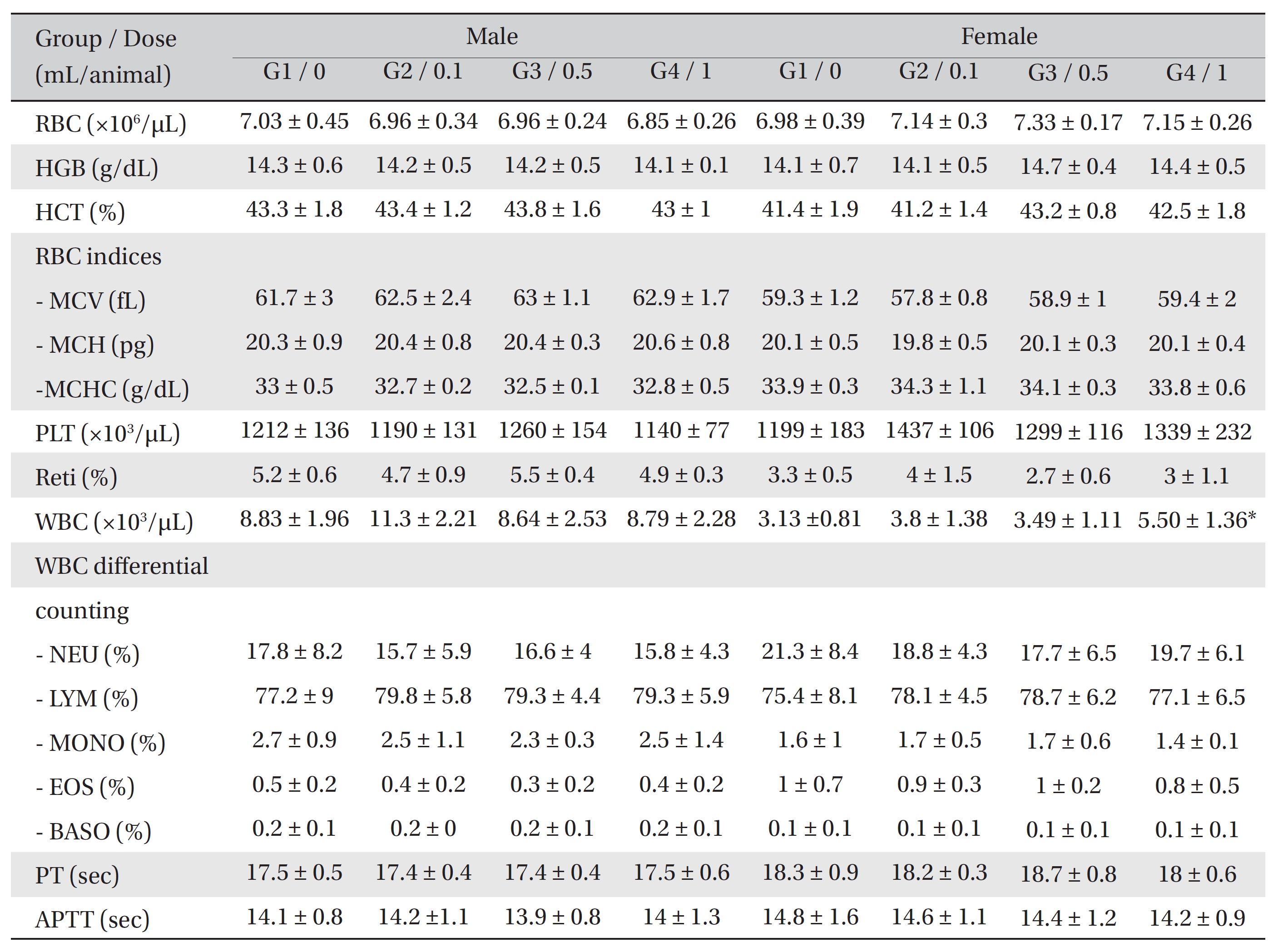
[Table. 2] Hematological Values of SD Rats
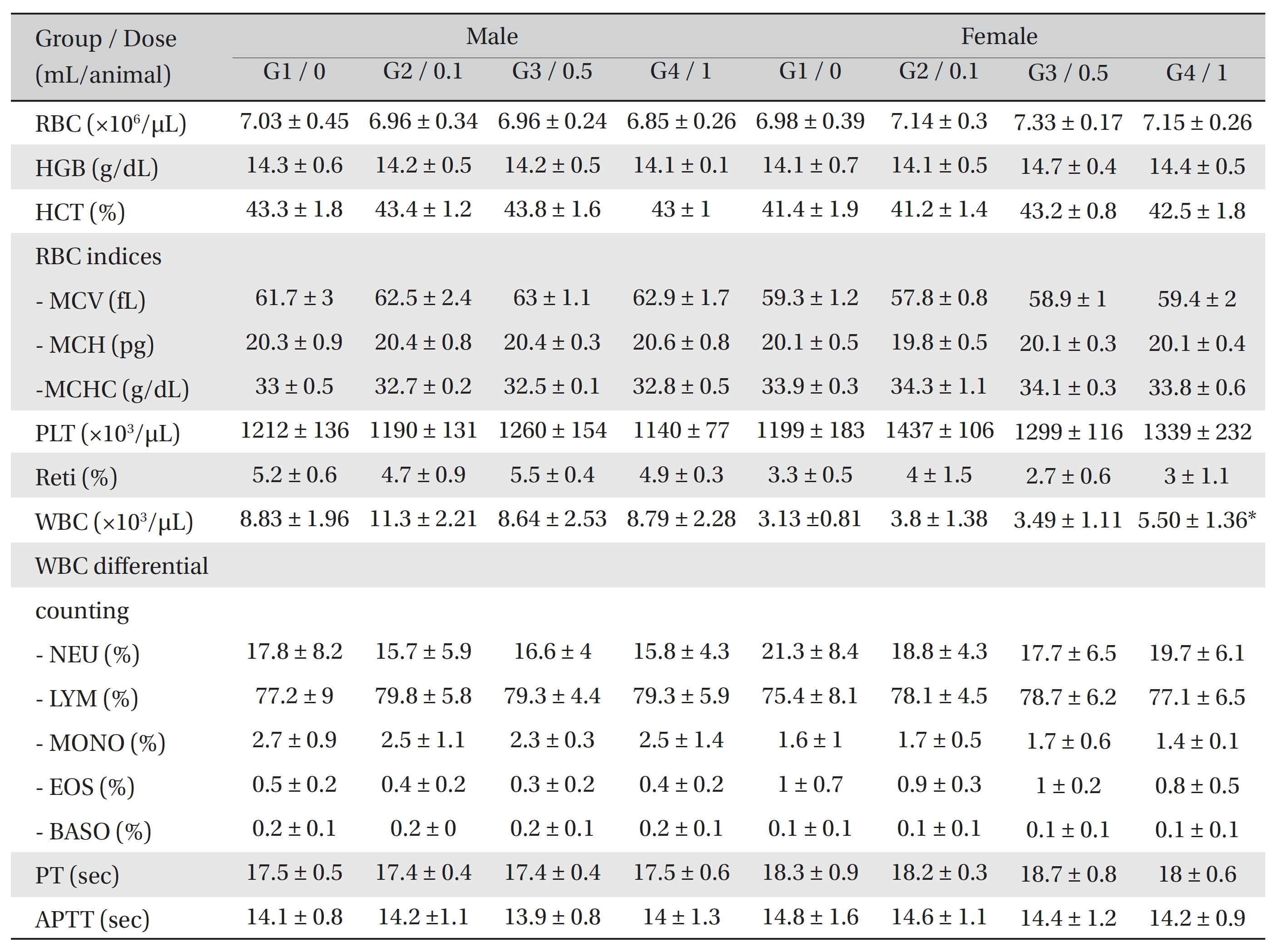
Hematological Values of SD Rats
During the observation, no deaths or abnormalities were observed in either the control or the experimental groups. However, crust formations were observed in the left ear, left neck, and left shoulder of one female of the control group.
The body weights in both the control and the experimental groups continuously increased during the observation period, but no significant changes in body weight were observed.
In the hematological test, the WBC counts were significantly increased in the female rats of G4 compared to G1.
However, no significant changes were found between the experimental groups and the control group (Table 2).
No significant biochemical changes were observed when the results of the experimental groups were compared with those of the control group.
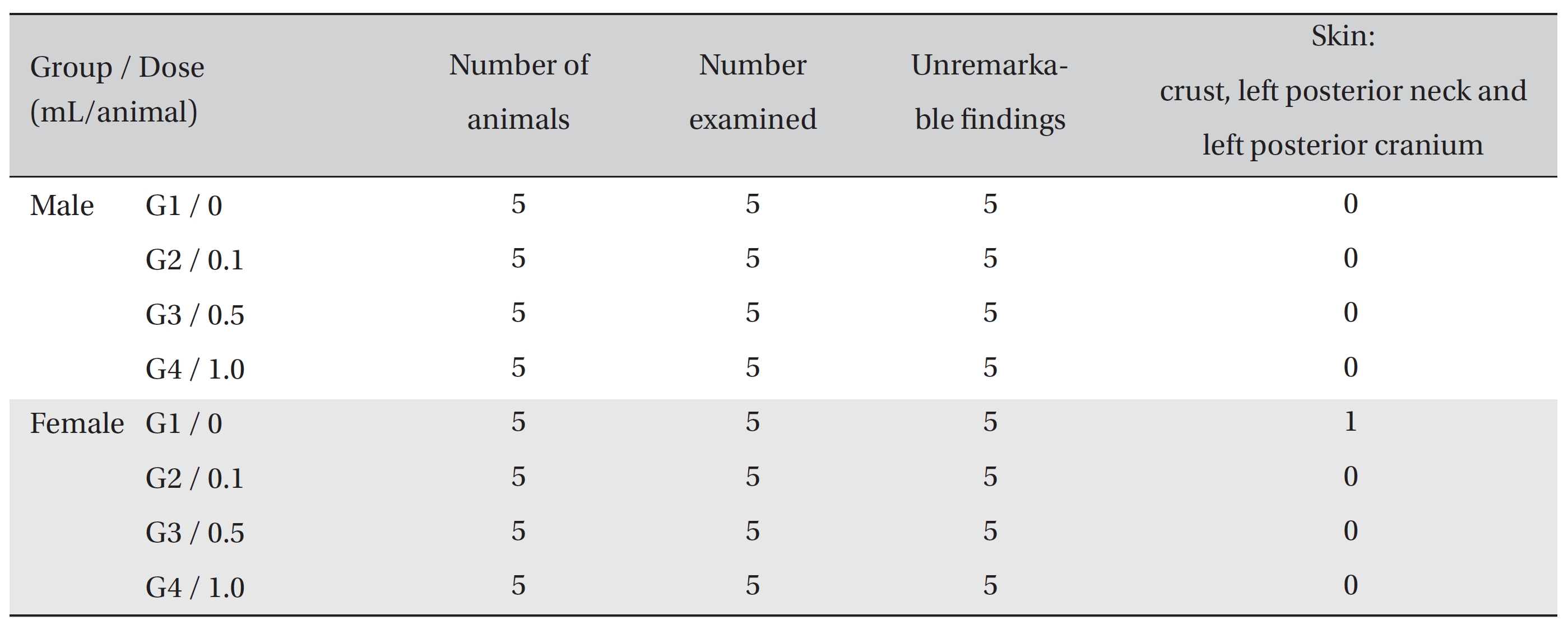
On necropsy, crust formation was observed in the posterior neck and the left posterior cranium of one female rat of the control group. However, no abnormalities were observed when visual inspections were conducted on both the control group and the experimental groups (Table 3)
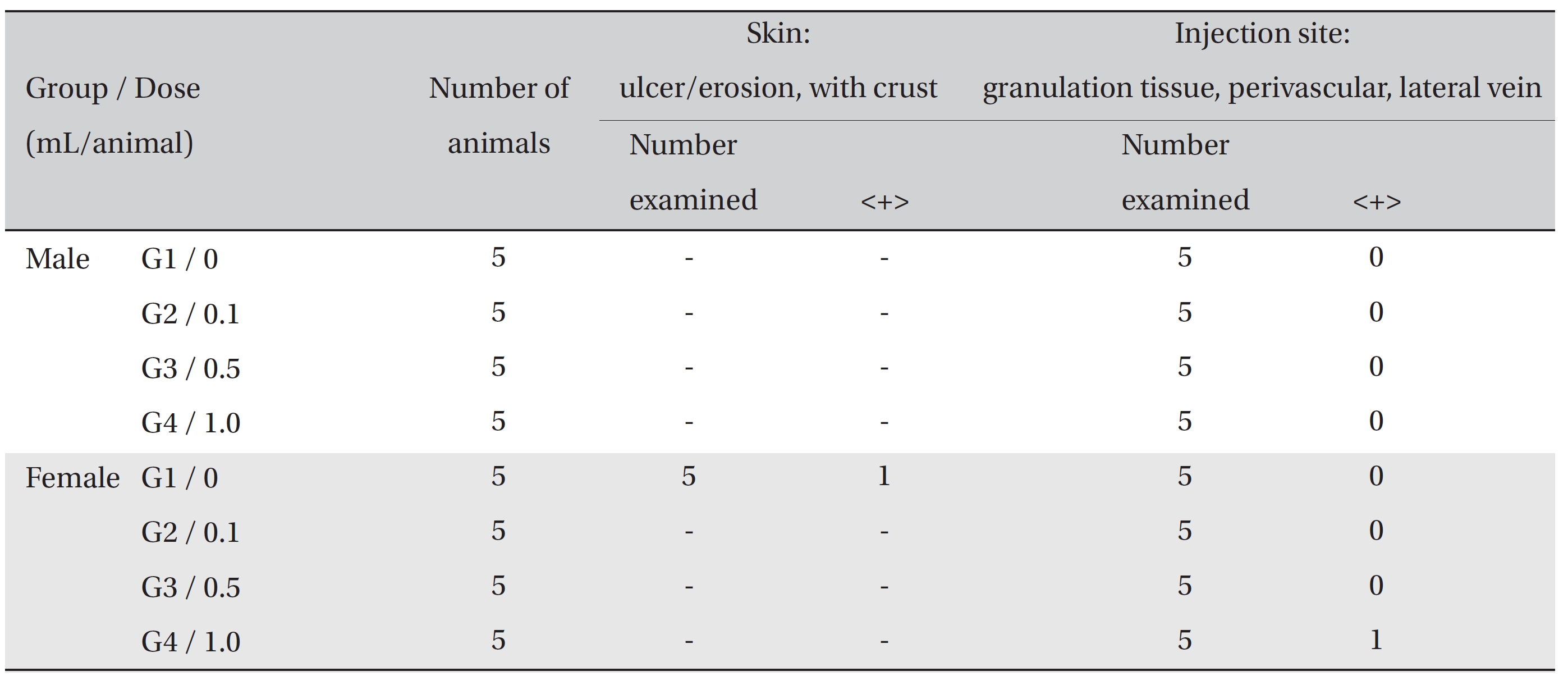
In the tolerance test on the injection sites, the crust found in the one female rat of the control group was confirmed, in the autopsy, to be an ulcer/erosion with crust. Granulation tissues were observed around the injection site - perivascular area of the lateral vein - of one female rat of G4. However, no abnormal histopathological signs were observed in either the control or the experimental groups (Table 4).
[Table. 3] Summary of necropsy findings
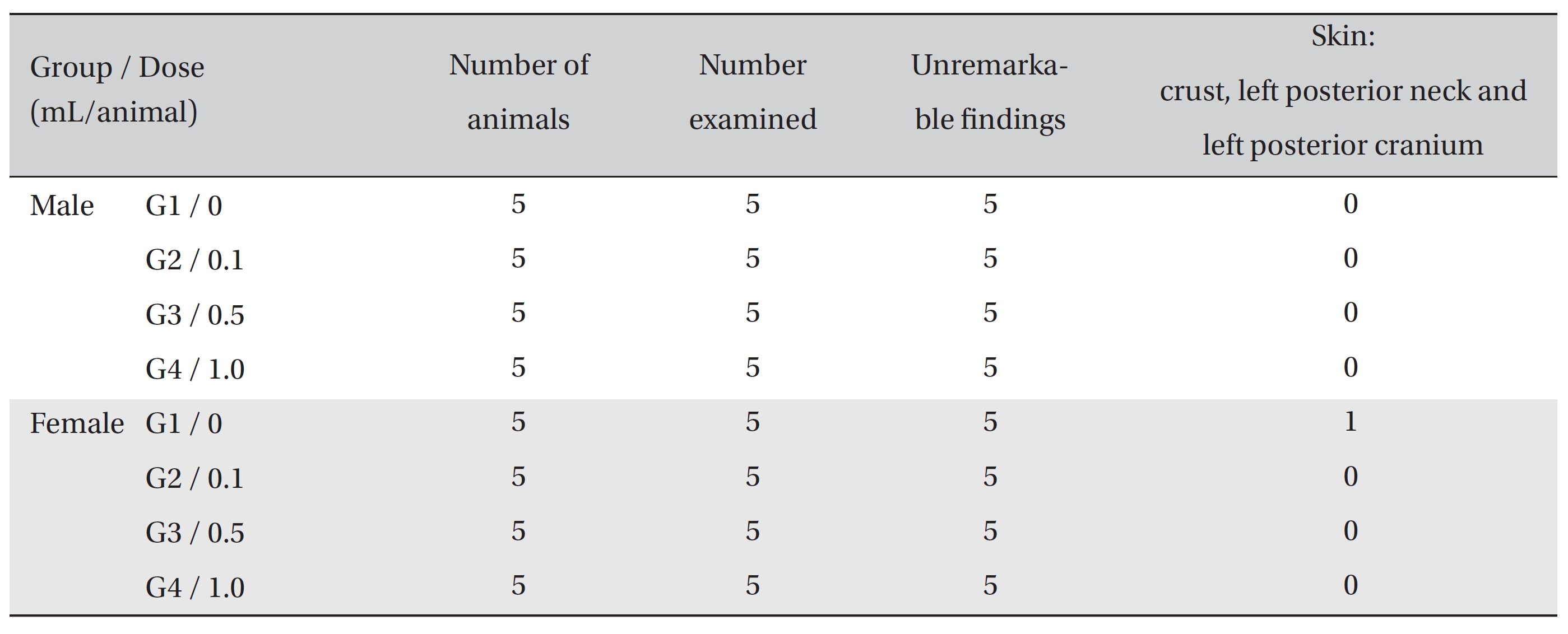
Summary of necropsy findings
[Table. 4] Summary of Histopathological Findings
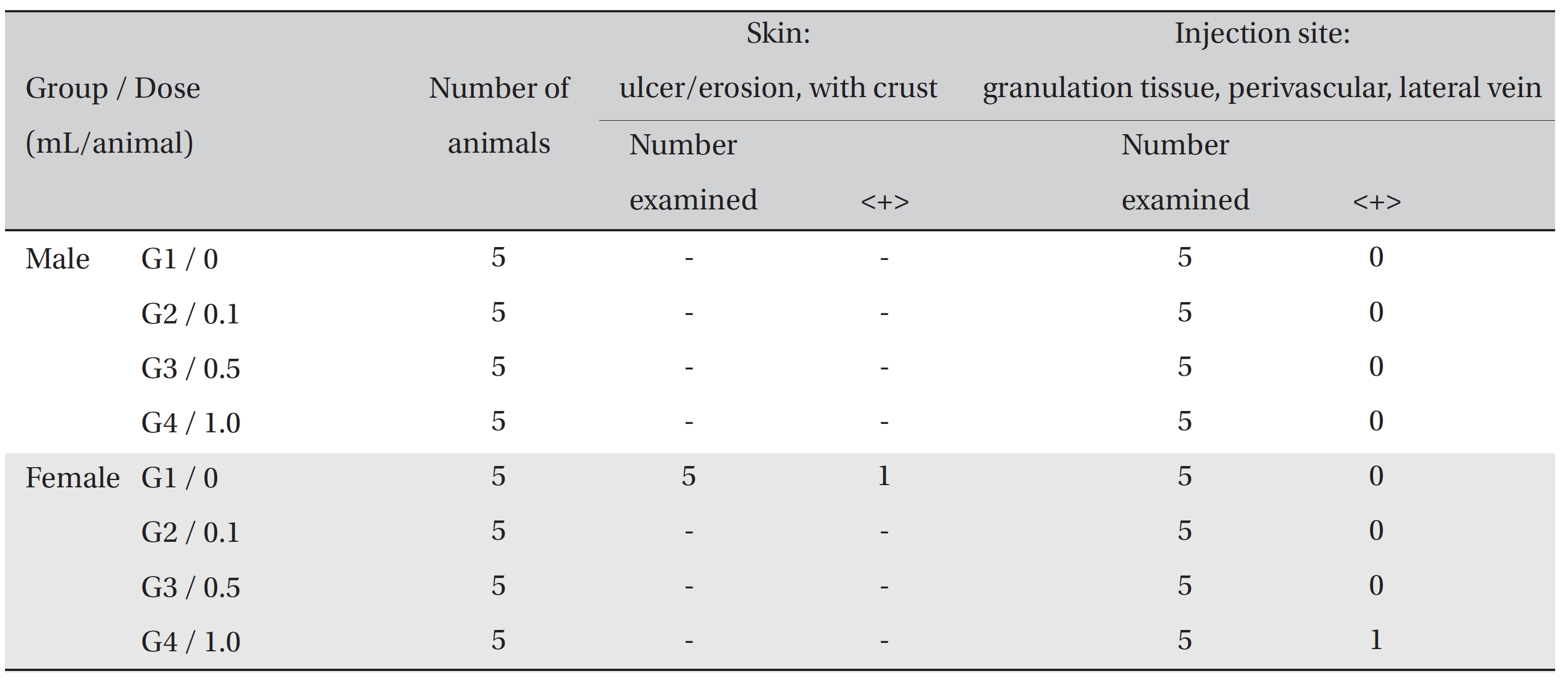
Summary of Histopathological Findings
A toxicant refers to any substance that may harm the body in a sufficient concentration. Swiss scientist Paracelsus said, “All substances are poisons; there is none which is not a poison. The right dose differentiates a poison and a remedy” [7]. Therefore, a toxicity test should be conducted to prove the safety of a drug before its clinical use because any drug with minimum side effects can be toxic, depending on the dose.
There are three different types of toxicity tests: an acute toxicity test injecting a single-dose, a sub-acute toxicity test injecting a dose repeatedly for one month, and a chronic toxicity test injecting a dose repeatedly for over three months [8]. The toxicity test has recently been perceived as being better for focusing on finding the relationship between the dose and toxicity changes before and after the administration than for simply acquiring LD50 values [9]. The safety of a pharmacopuncture with respect to its toxicity needs to be proven because a pharmacopuncture uses an injection route, which is different from the oral administration route used in previous treatments.
Ju
Therefore, it can be utilized for clinical purposes because it is expected to have effects on immunity reinforcement, and obesity prevention and to have minimum side effects. However, toxicity tests in animals have not been reported in Korea yet.
In China, injectable formulations of Chinese medicine such as Houttuyniae Herba, Bupleuri Radix, Astragali Radix, and Radix Salviae Miltiorrhizae Composita were made and administered through intravenous injection to animals [11]. Many studies have been conducted in various areas such as clinical treatment [12] and side-effect management [13].
However, pharmacopuncture studies in Korea are mainly conducted via subcutaneous or intramuscular injection through an acupoint or a tender point. Studies of intravascular pharmacopuncture injection have rarely been conducted, the only exception being a study on wild Ginseng Radix pharmacopuncture [9, 14], because of the limited use of intramuscular injection in medical practice in Korea. Therefore, a toxicity test for
SD rats have been widely used in safety tests of drugs and related materials because of the system of their supply is stable and because they have consistent reactions to drugs [15]. Thus, they are considered as appropriate for use as test animals.
No deaths from toxicity occurred as a result of
In the hematological test, female rats of G4 showed a significant increase in WBC counts compared to the control group. However, the result demonstrated a small variance and showed no dose-dependency or consistent variance by sex. Thus, the result was not considered to be statistically meaningful. No significant changes were observed in the biochemical test when the results from the experimental groups and the control group were compared.
On autopsy, a crust was observed with the naked eyes on the posterior neck and the cranium of one female rat of the control group. It was confirmed to be an ulcer or erosion, but it was concluded to be a one-time or accidental change. In the local tolerance test, granulation tissue was observed around the injection site in one female rat of G4. However, the formation of granulation tissue is a general mechanism of wound healing, as are angiogenesis and increased vascular permeability [16]. The formation of granulation tissue was observed in only one individual, so it was concluded to be due to changes caused by insertion of the needle into the vein. No other results were found in the autopsy and the local tolerance test on the injected sites.
A single-dose of
Further studies should be conducted to evaluate the toxicity more accurately for clinical applications. Also, toxicity tests for non-rodents and sub-acute or chronic toxicity tests with repeated administration should be conducted.
A single-dose of