This study evaluated the single-dose toxicity of Saeng Maek San (SMS) in rats.
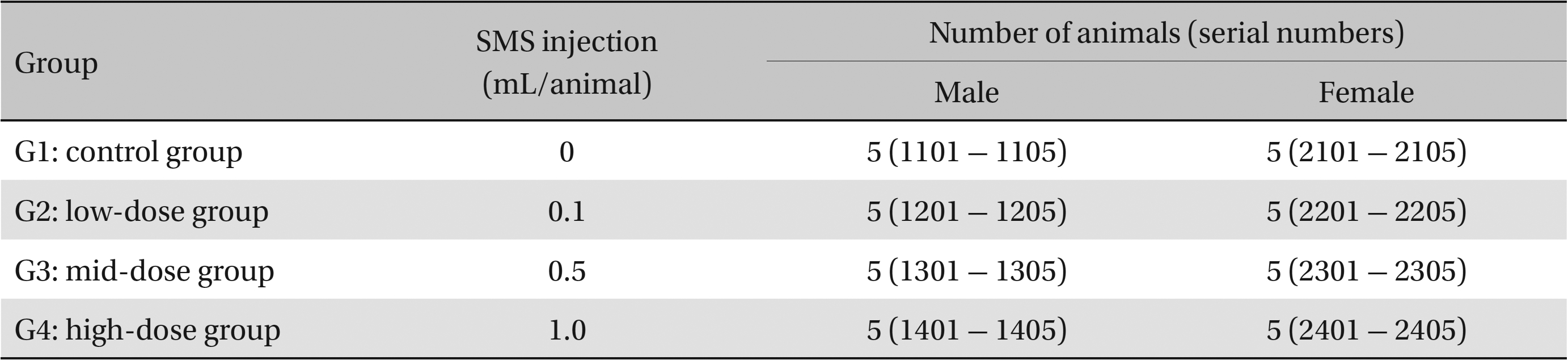
All experiments were conducted at Biotoxtech (Chungwon, Korea), an institute authorized to perform non-clinical studies under the regulations of Good Laboratory Practice (GLP). A single-dose intravenous toxicity study was carried out on 40 6-week-old Sprague-Daley rats. The animals were randomly divided into the following four groups of ten animals each: Group 1 (G1) was the control group, with each animal receiving an intravenous injection of 1.0 mL of saline, and Groups 2, 3 and 4 (G2, G3 and G4) were the experimental groups, with the animals in the groups receiving an injection of 0.1, 0.5 and 1.0 mL of SMS, respectively. Mortality, clinical signs, body-weight changes and gross pathological findings were observed for 14 days following a single administration of SMS or saline. Organ weights, clinical chemistry and hematology were analyzed at 14 days. This study was conducted with the approval of the Institutional Animal Ethics Committee.
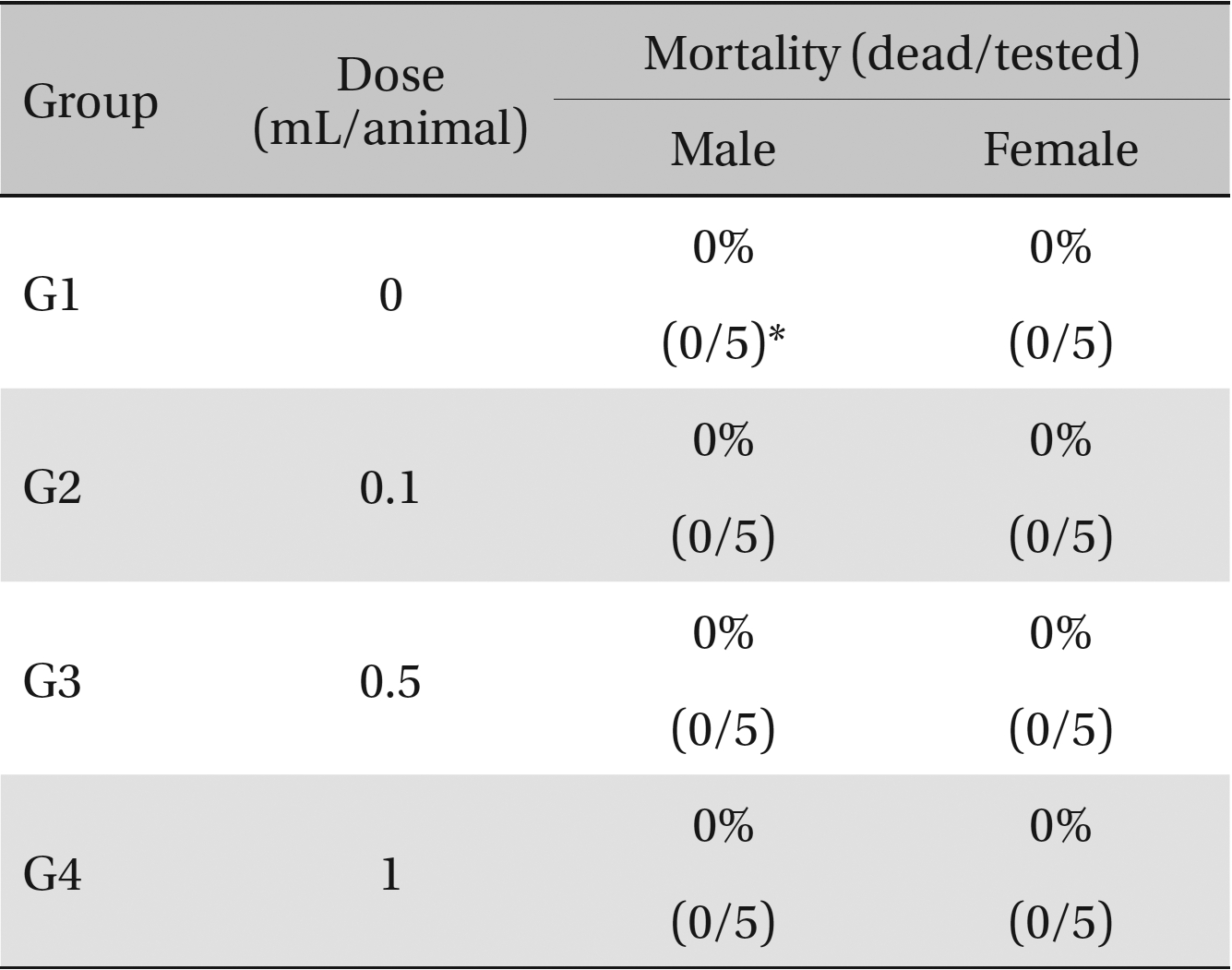
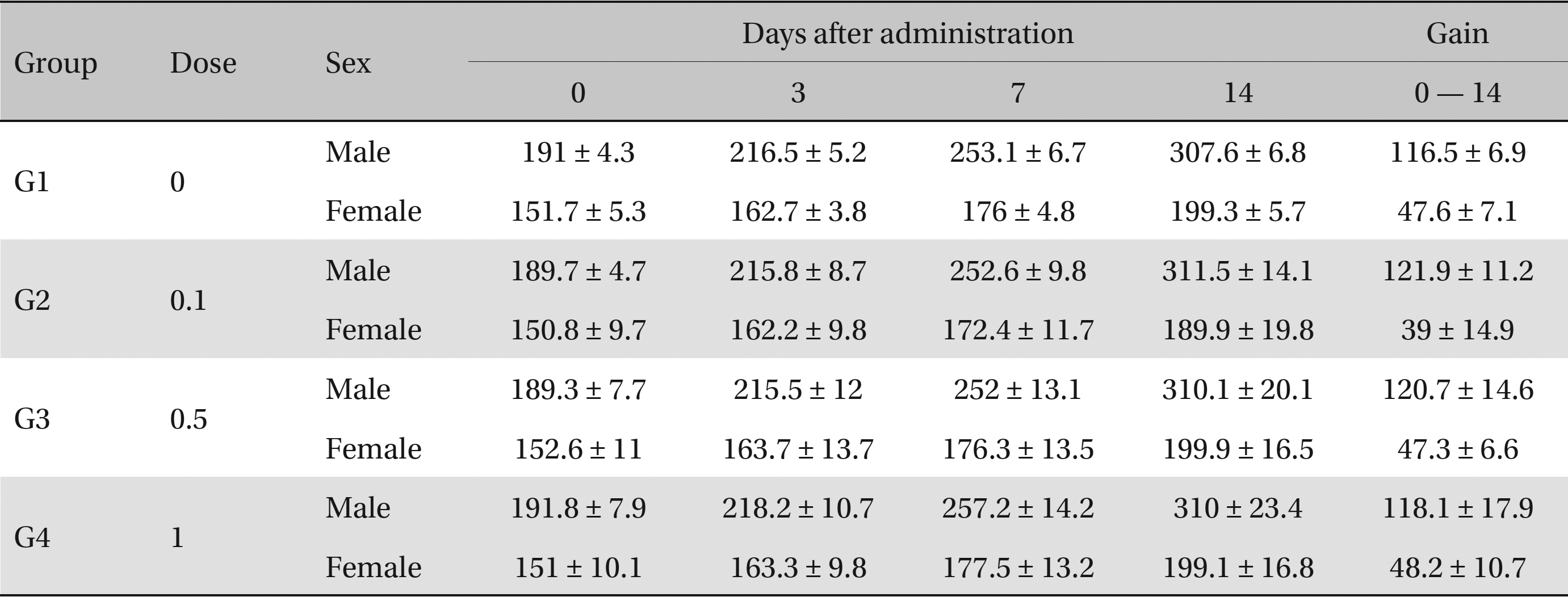
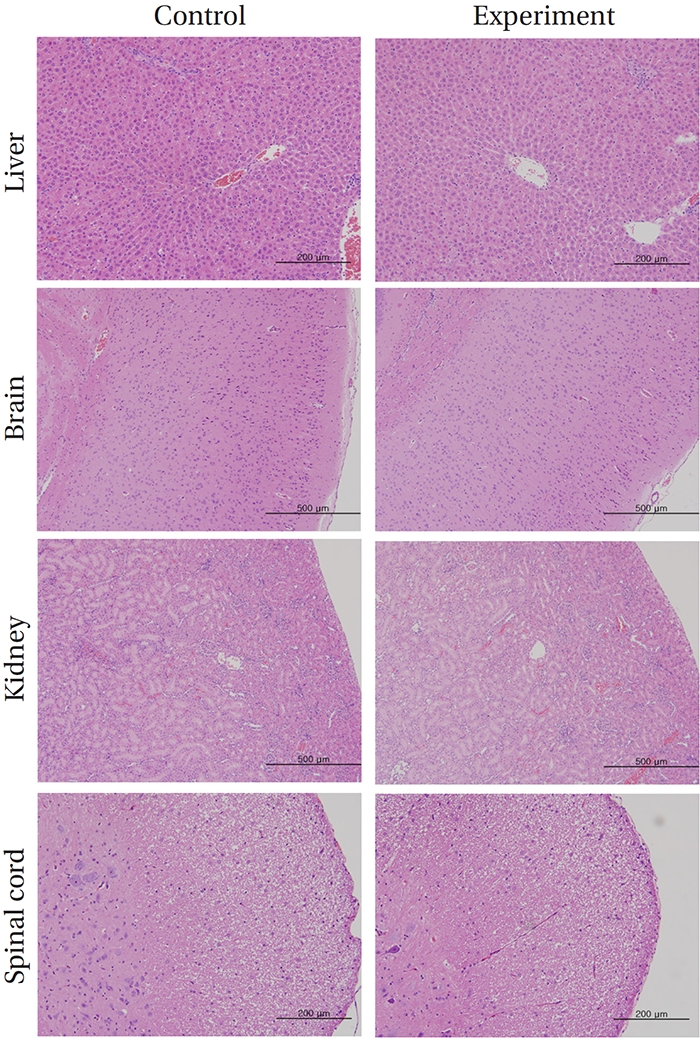
No deaths occurred in any of the four groups,indicating that the lethal dose of SMS in rats is greater than 1.0 mL/animal. Some changes in weights of male rats between the control group and the experimental groups were observed, but no significant changes in the weights of female rats were noted. To identify abnormalities in organs and tissues, we stained representative sections of each specified organ with hematoxylin and eosin for examination with a light microscope. No significant abnormalities were observed in any of the organs or tissues.
The results suggest that intravenous injection of SMS is a safe method of treatment.
Saeng Maek San (SMS), a traditional Korean herbal medicine, composed of
In an
Despite the efficacy and the non-toxicity of SMS, there has to date been no detailed investigation of the acute toxicity of such a commonly used drug. To build upon the above-mentioned studies, the safe concentration of SMS in the blood should be determined. This study provides data regarding both the median lethal dosage (LD50) of SMS in rats through different routes of injection and the largest safe dose for intravenous injection.
SMS, which is a mixture of three traditional herbs, was prepared under sterile conditions at the Korean Pharmacopuncture Institute (K-GMP). The ratio of the herbal drugs in SMS is 2 : 1 : 1 of Liriopis Tuber, Ginseng Radix and Schisandrae Fructus, respectively (total: 5500 g). SMS extract was prepared by decocting the dried prescription in distilled water. The extract was obtained by decocting for approximately 2 hours (total extracts: 12 L), and the pH was controlled to between 7.0 - 7.5 by adding NaOH to make a 0.9% isotonic solution. The final solution was stored at 4°C.
This study used 5-week-old male and female Sprague-Dawley rats (supplied by Orient Bio Inc., Korea) weighing 103.4 - 134.1 g. Sprague-Dawley rats are used widely for drug-safety testing. The animals’ weights at the time of injection were 180.4 - 202.2 g and 135.4 - 165.7 g for the male and the female rats, respectively. The general condition of, and any symptoms displayed by, the rats were observed once per day for 7 days following injection; the weights of the rats on day 7 were recorded. The room in which the rats were kept was maintained at 20.0 - 22.8°C, with a relative humidity of 48.5% to 65.9%, natural illumination, and automated ventilation. The animals were housed individually in cages and allowed free access to solid feed (Teklad Certified Irradiated Global 18% Protein Rodent Diet 2918C) and UV-filtered water. Groupings of the rats were allocated 7 days after injection. Animals with weights close to the mean were selected. In total, 20 male and 20 female rats were selected. The animals were randomly distributed into four groups of five rats each (Table 1).
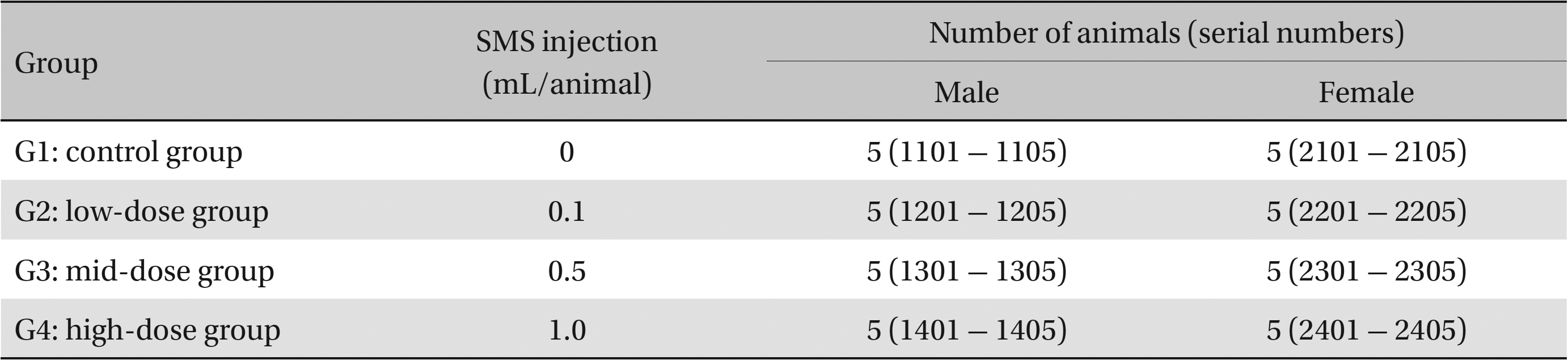
Groups of animals
In clinical applications, the standard dose for SMS is 1.0 mL per treatment. In our study, 1.0 mL/animal was set as the high dose and 0.5 and 0.1 mL as the medium and the low doses, respectively. Normal saline (1.0 mL) was administered to control rats. A single dose of SMS at the prescribed level was injected intravenously into the rats in the low-, the medium-, and the high-dose experimental groups. Clean disposable syringes were used throughout the study. The study was conducted with the approval of the Institutional Animal Ethics Committee (No.120877).
General symptoms were recorded daily at 1 to 14 days following injection. On the day of dosing (Day 0), general symptoms (side effects, revealing time, recovery time, etc.) and mortality were examined at 30 minutes and at 1, 2, 3 and 4 hours after injection. The weight of each rat was measured immediately before injection and at 3, 7, and 14 days thereafter.
Fifteen days after injection on the day of necropsy, after the rats had been fasted for at least 18 hours, they were anesthetized with isoflurane, and a blood sample was taken from the abdominal aorta). Blood (1.0 mL) was analyzed using an automatic hematology analyzer (ADVIA 120, SIEMEMS, Germany). Blood coagulation was assessed by centrifuging a 2.0-mL blood sample (3,000 rpm, 10 minutes) and taking measurements using an Automated Coagulation Analyzer (Coapresta 2000, SEKISUI, Japan). Biochemical tests were performed on blood taken from the abdominal aorta by using an Automatic Analyzer (7180, HITACHI, Japan) and Electrolyte Analyzer (AVL9181, Roche, Germany).
After completion of the above measurements, all organs and tissues of each rat were inspected visually and were examined under an optical microscope. The organs of each rat were fixed with 10% formalin or Calci-Clear-RapidTM solution (the latter only for testes showing osseous tissue demineralization), stained with hematoxylin–eosin (H&E) and fixed in paraffin wax. A complete histopathological examination was performed on these tissue samples.
The weight, hematological, and blood biochemical test results were analyzed using the statistical analysis system (SAS) software (versions 9.2 and 9.3, SAS Institute Inc., USA). A Bartlett’s test was conducted to evaluate the homogeneity of the variance and significance. A oneway analysis of variance (ANOVA) was conducted when homogeneity of the variance was recognized, and the Kruskal-Wallis test was conducted as a post-hoc analysis.
3. Results
No deaths or abnormalities occurred in any of the groups, indicating that the LD50 of the SMS administered via intravenous injection was greater than 1.0 mL/animal in rats (Table 2, 3). In addition, no changes in weight were observed (Table 4). In this study, even though computation of the LD50 was difficult, all groups presented a sustained increase in body weight, but the differences among the groups were not significant. Finally, no significant changes in the hematological results, blood biochemical parameters or necropsy findings were noted (data not shown). Upon histopathological examination, interstitial- infiltrating macrophages were found in one female rat in the 0.5-mL/animal group. No significant changes in the brain, lungs, liver, kidney or spinal cord were found in any of the other groups (Fig 1).
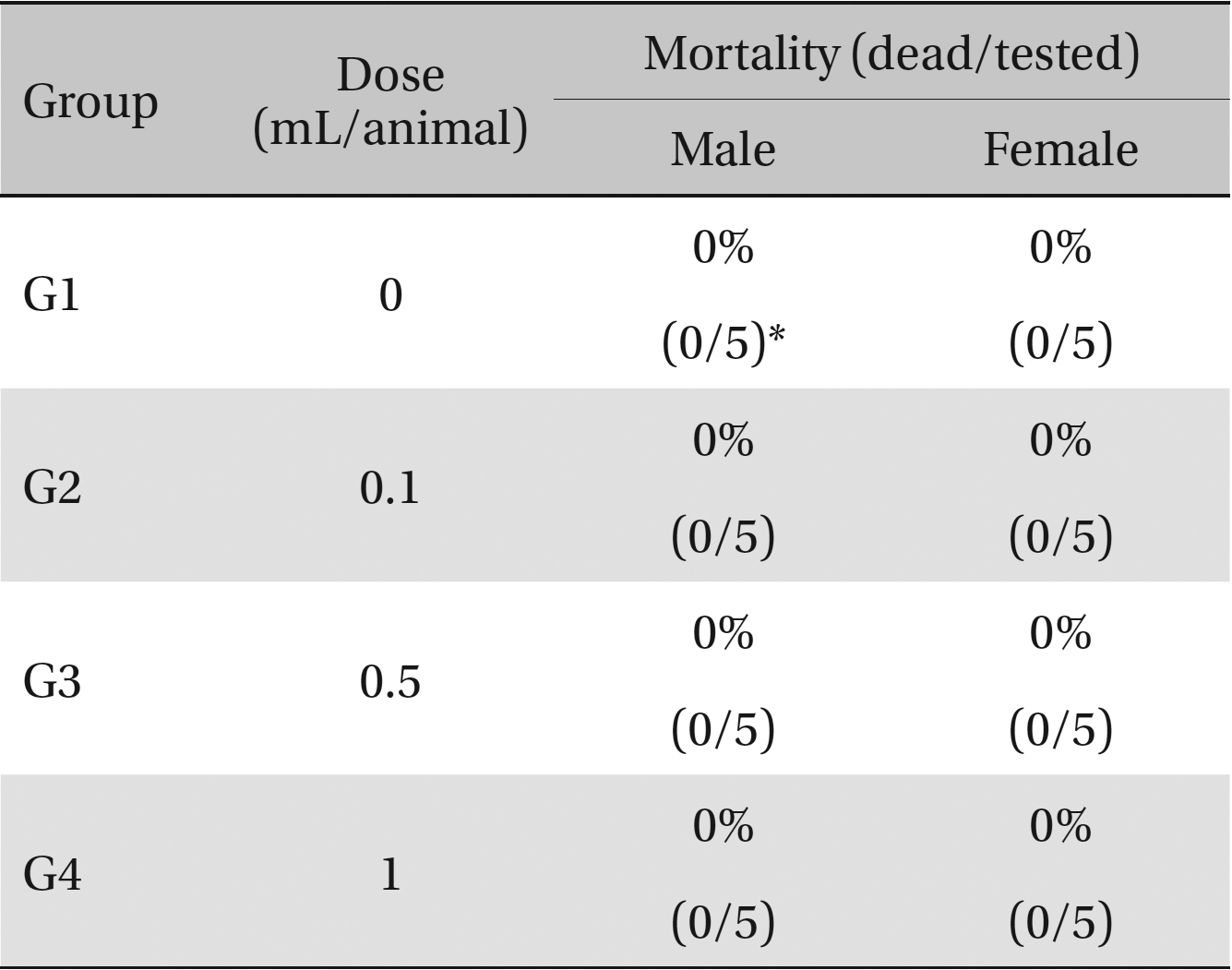
Mortalities. A single intravenous injection dose of SMS at the each groups including 1.0 mL/animal group caused no mortalities.
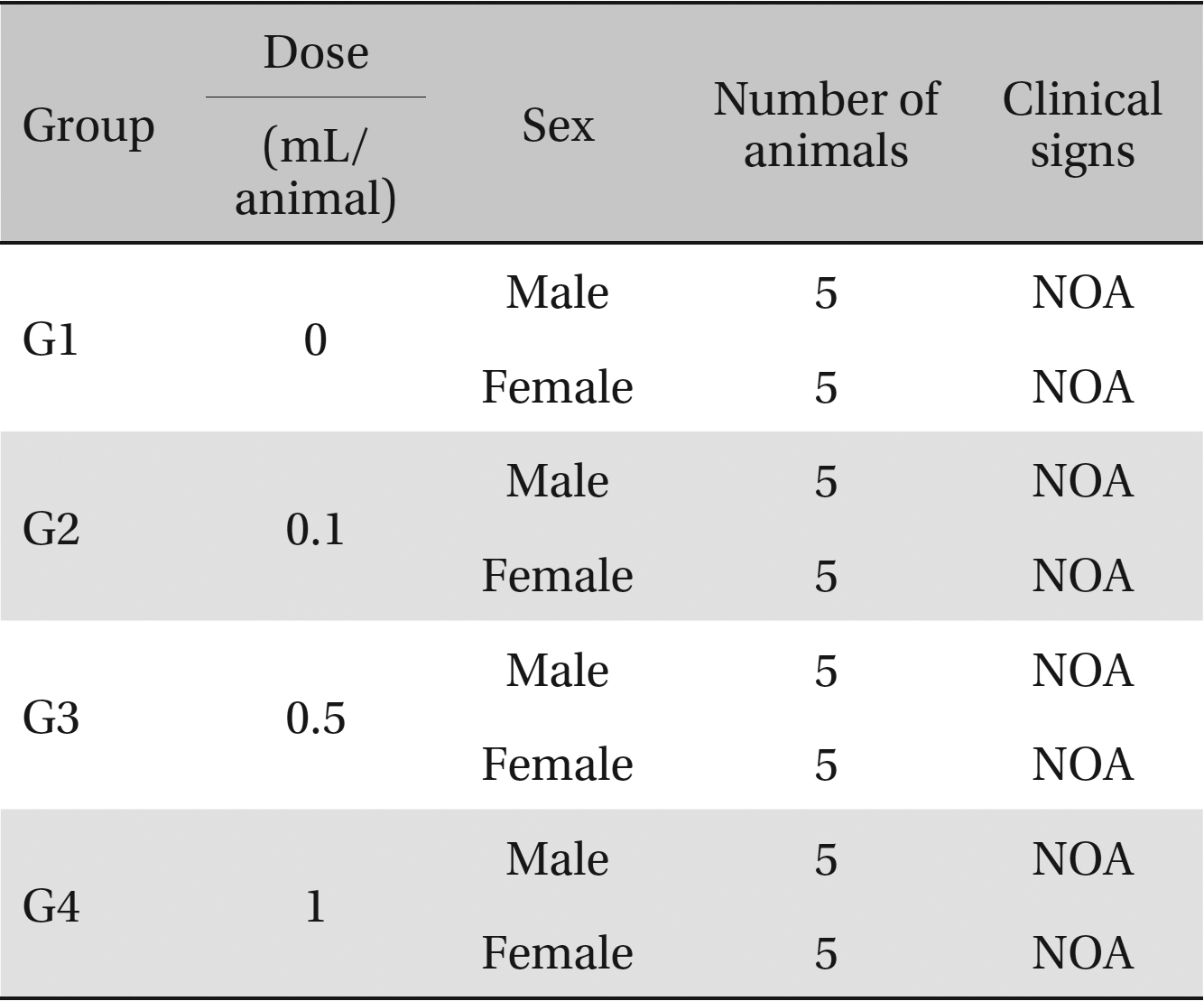
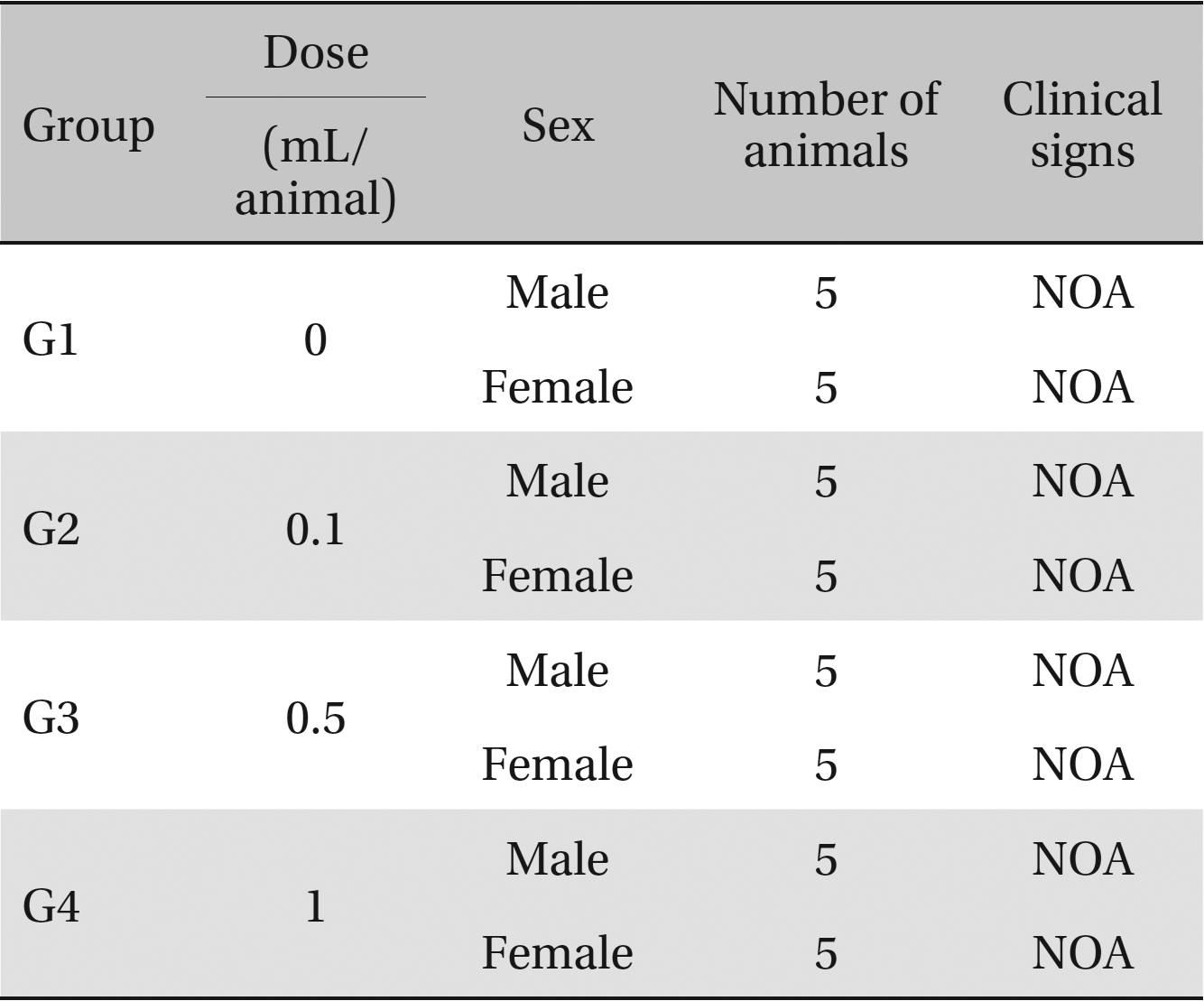
Clinical signs. No abnormal clinical symptoms, such as type of toxicity symptom, time of expression, time of recovery were observed after injection.
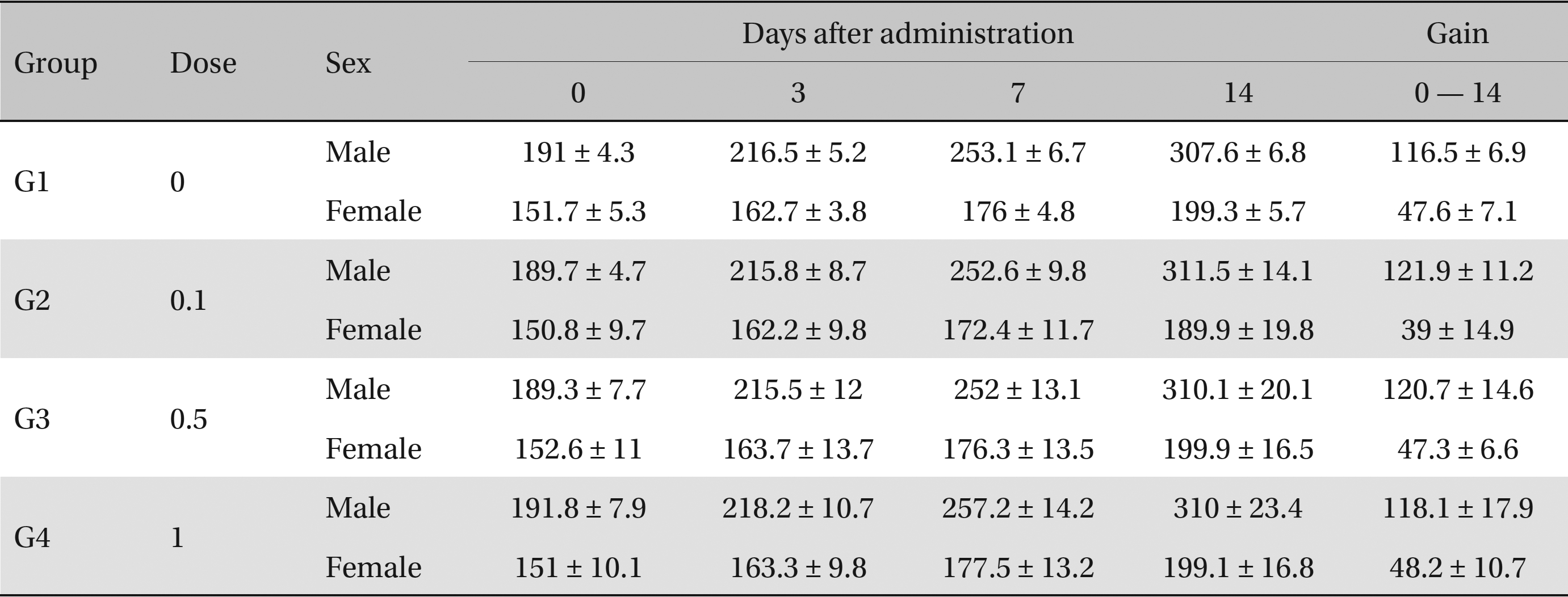
Body weights (g)
SMS is a traditional medical formula composed of
Thus, SMS and its component herbs have been reported to have a myriad of positive effects on several disorders. Although it is used widely in clinics, studies on the safety of SMS are lacking, so further research is needed, and toxicity testing is essential for evaluating the safety of medications [29]. This study was performed to provide objective safety data for SMS. Three SMS doses (0.1, 0.5 and 1.0 mL) were administered to the experimental groups, and 1.0 ml of saline was administered to the control group. In all four groups, no deaths occurred, indicating that the LD50 of SMS is greater than 1.0 mL/animal in rats. There were no significant differences between the control group and the experimental groups in the clinical signs, weights, hematologic examination results, and blood biochemical parameters. At necropsy, only one rat displayed any significant histopathological abnormalities in the organs and tissues.
Further studies of the acute and the chronic side effects and the reaction capacity are required to rigorously assess the toxicity of SMS. Animal testing is the most important method of performing these safety assessments [30]. The Korea Food and Drug Administration has testing-protocol guidelines for the assessment of toxicity, and all experiments should be conducted following Good Laboratory Practice (GLP) regulations [31]. In this study, the LD50 of SMS in rats was shown to be > 1.0 mL/animal, indicating that this dose is safe in humans and does not cause significant histological abnormalities. Further studies are needed to yield more data to support our findings.
In our study, the administration of 1.0 mL/animal SMS did not produce any significant changes in body weight, in the results of hematological, blood biochemistry or necropsy examinations, or in the incidence of mortality. Our findings indicate that SMS administration up to this dose is a safe option for treatment.