This study was performed to analyze the single-dose toxicity of neutral natured blood stasis pharmacopuncture extracts.
All experiments were conducted at Biotoxtech, an institution authorized to perform non-clinical studies, under the regulations of Good Laboratory Practice (GLP). Sprague-Dawley rats were chosen for the pilot study. Doses of neutral natured blood stasis pharmacopuncture extracts, 0.1, 0.5 and 1.0 mL, were administered to the experimental group, and the same doses of normal saline solution were administered to the control group. This study was conducted under the approval of the Institutional Animal Ethics Committee.
In all 4 groups, no deaths occurred, and the neutral natured blood stasis pharmacopuncture extracts administered by intramuscular (IM) injection was over 1.0 mL/animal. No significant changes in the body weights between the control group and the experimental group were observed. To check for abnormalities in organs and tissues, we used microscopy to examine representative histological sections of each specified organ; the results showed no significant differences in any organs or tissues.
The above findings suggest that treatment with neutral natured blood stasis pharmacopuncture extracts is relatively safe. Further studies on this subject should be conducted to yield more concrete evidence.
Recently, toxicity and side effects in the human body have been increasing due to unsafe foods and the drug abuse. Thus, close inspection and regulation are needed [1]. Pharmacopuncture is one form of neo-acupuncture based on meridian theory. In pharmacopuncture, an injection is made of several herbal medicines in a regular way at correlated acupuncture points, tender points, and positively-reacting points on the skin. The strong points of pharmacopuncture are the small dose needed for the treatment, the rapid speed at which it produces an effect, and its effectiveness. In addition, it is not destroyed in the gastrointestinal (GI) tract, and it is applicable with patients having difficulty swallowing [2, 3]. The weak point is that after the injection,an allergic reaction or localized skin stimulation or general body symptoms may occur, which worry the doctor and the patients in the clinic and reduce the popularity of pharmacopuncture [4].
Above all, the neutral natured blood stasis pharmacopuncture, which consists of
Studies such as the above on the effects of neutral natured blood stasis pharmacopuncture are ongoing, but toxicity tests have never been done. Accordingly, the authors have done toxicity tests and have obtained significant results for the toxic response and the approximate lethal dose of a one-time intramuscular (IM) injection of the neutral natured blood stasis pharmacopuncture for 6-week-old Sprague-Dawley (SD) male and female rats. Those results are reported here.
The current research trend for single-dose toxicity testing of extracts is to study acute and subacute toxicity using the procedures in the Good Laboratory Practice (GLP) [10]. All experiments for this research were conducted at Biotoxtech, an institution authorized to perform non-clinical studies, under the regulations of the GLP.
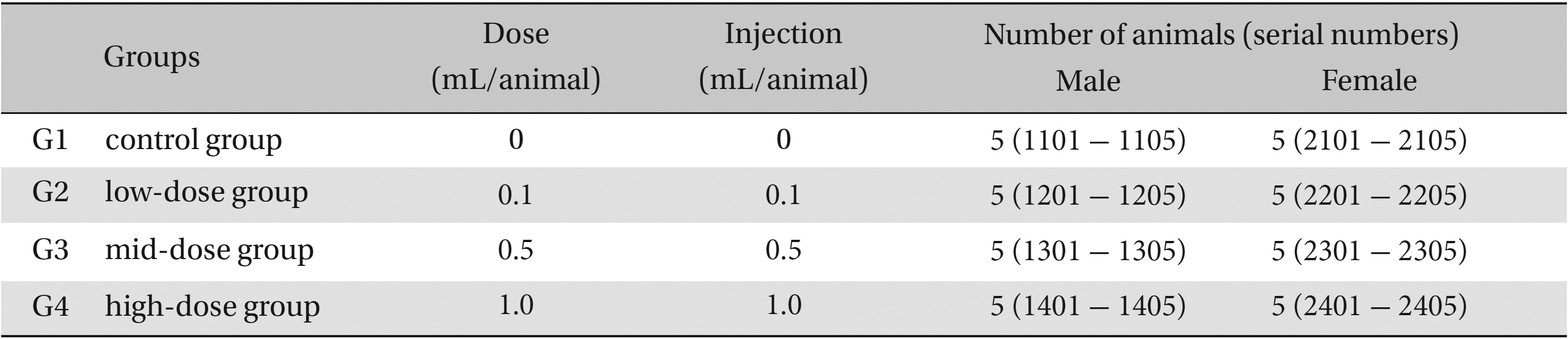
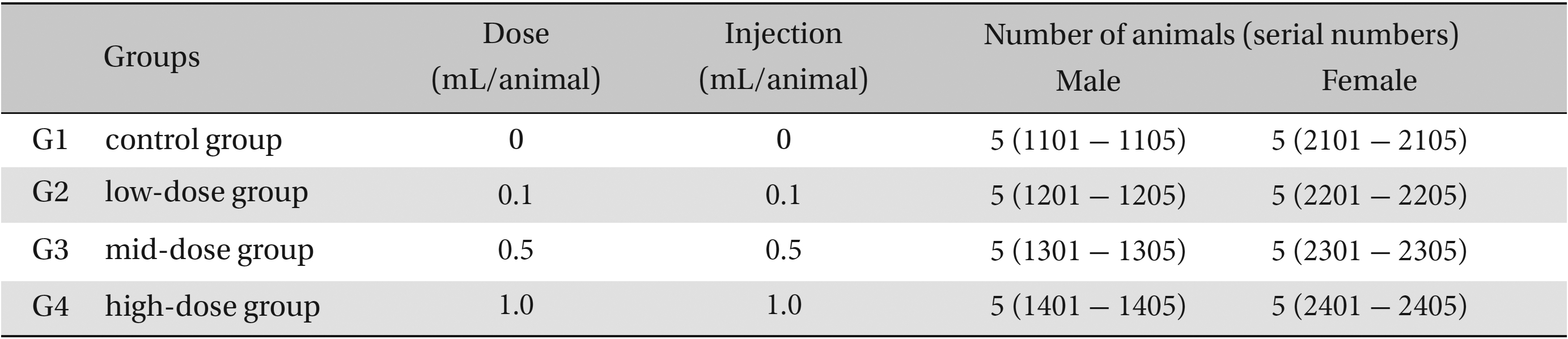
The neutral natured blood stasis pharmacopuncture (Korean Pharmacopuncture Institute, Seoul, Korea) extract was prepared by adhering to Korea-Good Manufacturing Practice (K-GMP) in a clean room in a lab at the Korean Pharmacopuncture Institute. It was stored in a refrigerator (2.1 - 6.3℃). The animals used in this study were 6-week-old Sprague-Dawley rats. The mean weights of the rats were 164.2 - 197.4 g, and 145.6 - 164.9 g for the male and female rats, respectively. For all animals, a visual inspection was done, and all animals were weighed using a CP3202S system (Sartorius, Göttingen, Germany). After 7 days of acclimatization, the rats’ general symptoms and changes in weight were recorded. The temperature of the lab was 19.6 - 23.1℃ and the humidity was 45.7% - 61.5%. Enough food (Teklad Certified Irradiated Global 18% Protein Rodent Diet 2918C) and UV-filtered water were provided. Groupings were done after 7 days of acclimatization. Animals were selected if their weights were close to the mean weight. In total, 20 male rats and 20 female rats were selected. The animals were distributed into 4 groups (5 mice per group) as shown in Table 1.
The doses for neutral natured blood stasis pharmacopuncture were 0.1, 0.5 and 1.0 mL/site/animal, which was determined by a preliminary study (Biotoxtech Study No.: B12870P). In the control group, the same dose of normal saline solution was administered into a specific point of the thigh muscle by IM injection. This study was conducted under the approval of the Institutional Animal Ethics Committee.
On the day of dosing (day 0), the general symptoms (types of toxic symptoms, revealing times, recovery times, etc.) and the mortality were examined 30 minutes, and 1, 2, 4 and 6 hours after the injection. From the 1st day to 14th day of treatment, the general symptoms were examined once a day. The weights were measured immediately before treatment and at 3, 7 and 14 days after treatment. After the termination of observation, all surviving animals’ organs and tissues were visually inspected and examined under a microscope.
The weight results from the experiment were analyzed by using SAS (version 9.2, 9.3, SAS Institute Inc., USA). A Bartlett test was conducted to evaluate the homogeneity of the variance and the significance. The one-way analysis of variation (ANOVA) test was conducted when homogeneity of the variance was recognized, and the Dunnett’s
Number of animals
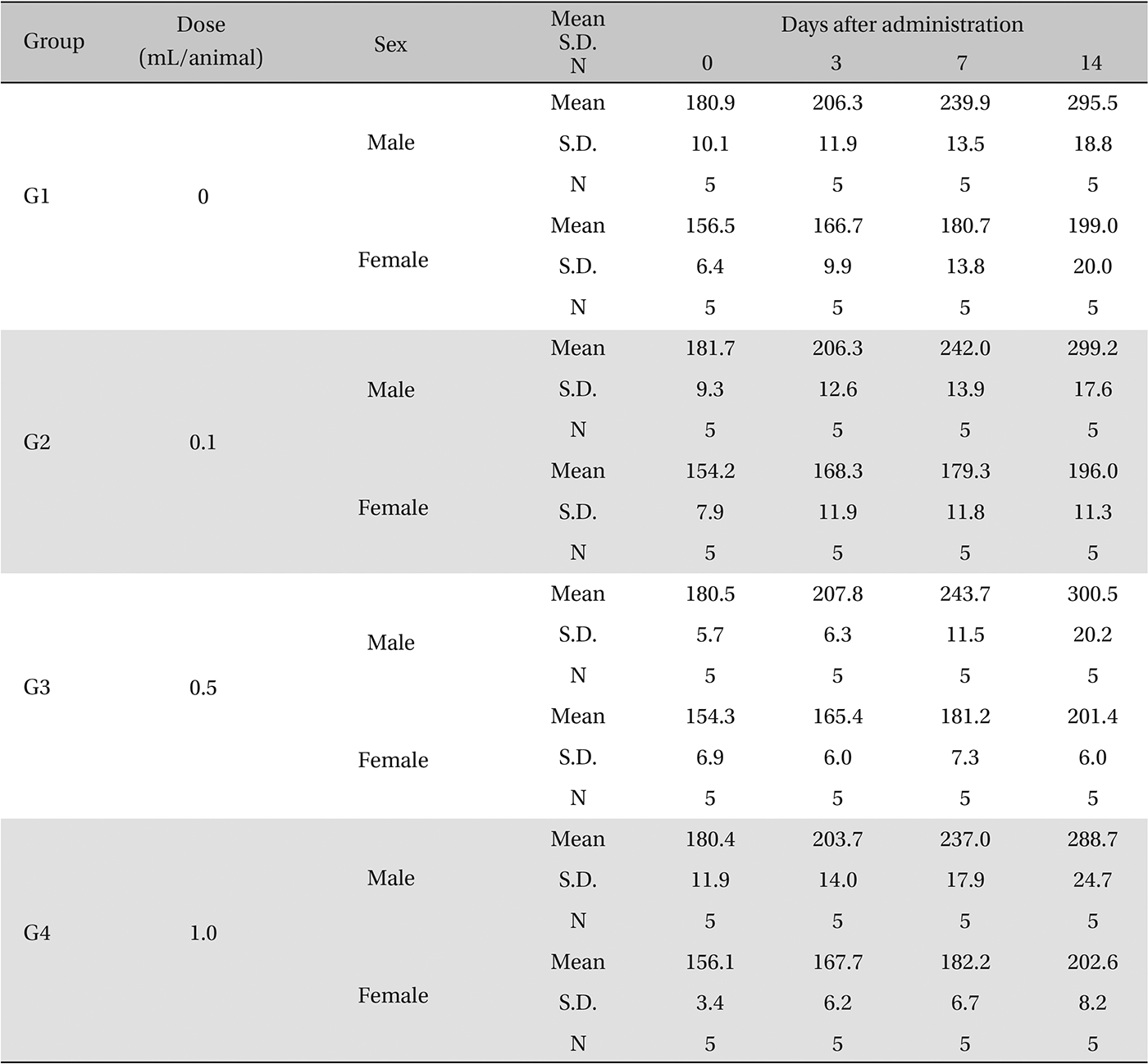
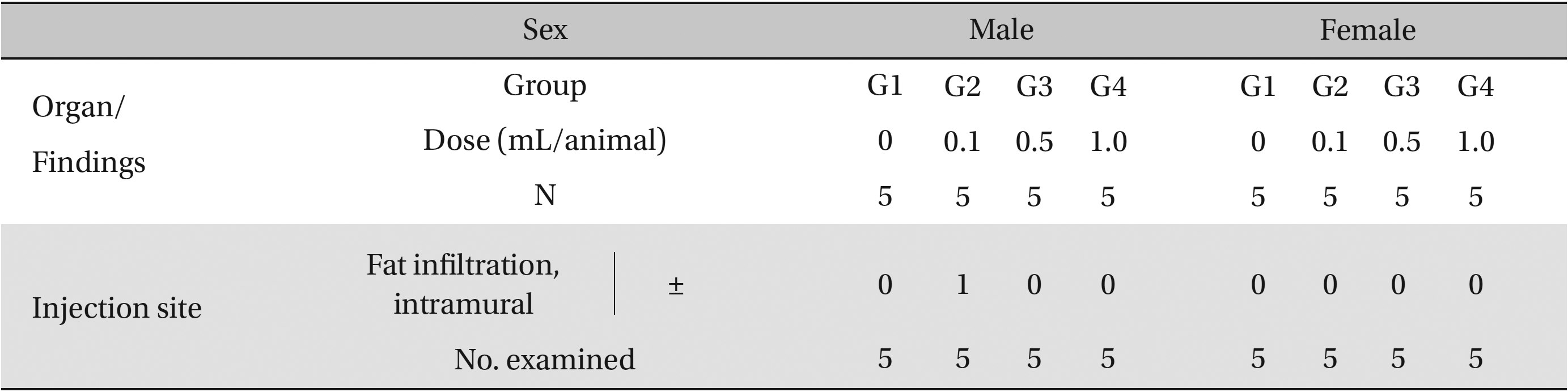
In this study, no deaths or abnormalities occurred in any of the groups, and the neutral natured blood stasis pharmacopuncture extracts administered via IM injection was over 1.0 mL/animal. In general, the body weights of both male and female rats increased with increasing treatment time, but no significance difference in body weights among the groups were noted at any particular times (Table 2). Also, on the hematologic and the blood biochemical test, the influence of extract injection could not be determined. Finally, no meaningful changes in necropsy were noted, and histopathological examinations of all groups found no significant changes related to injections in the brain, liver, kidneys and spinal cord; however, a male SD rat of G2 showed intramural fat infiltration, but it was minimal (Table 3).
[Table. 2] Body weights in grams
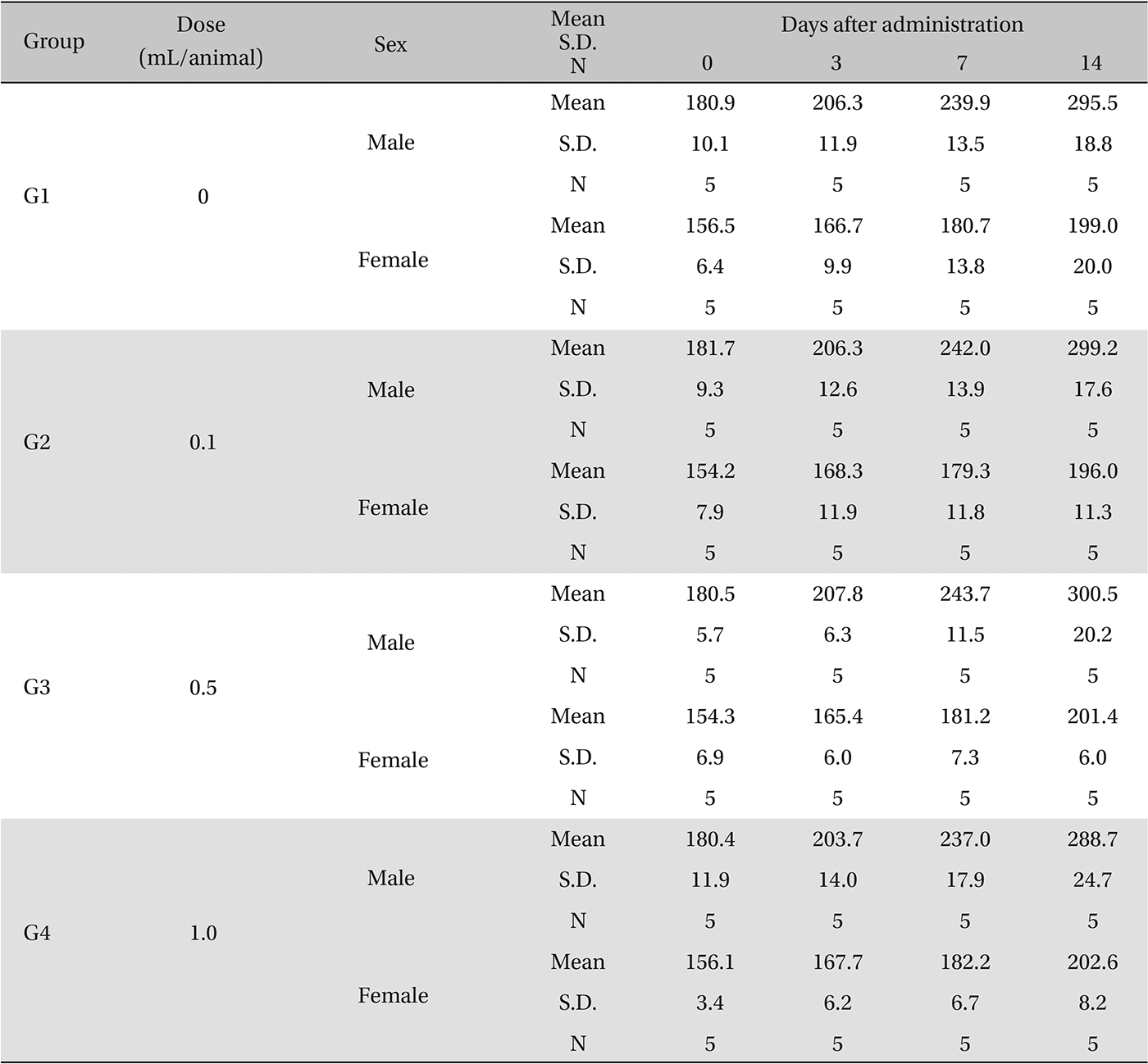
Body weights in grams
[Table. 3] Histopathological findings
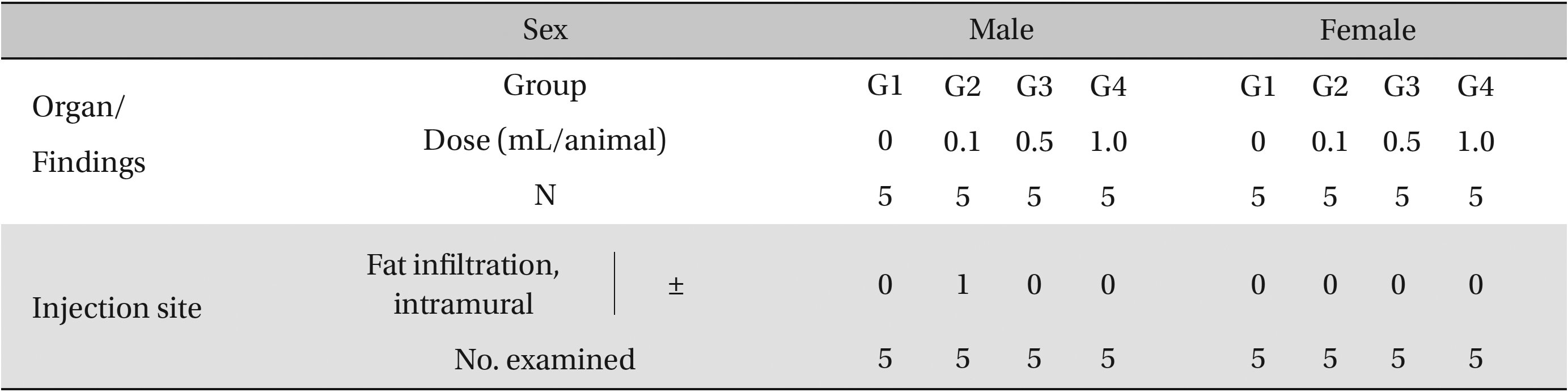
Histopathological findings
In research and development of herbal formulae, intra- laboratory verification of effectiveness is an important method to establish evidence-based Korean medicine [11]. A poison is a substance that can cause functional or morphological impairments to or have lethal effect on soft tissues if it comes into the human body via any of several routes [12]. Thus, in Korean medicine, herbal medicine is classified as
If the toxicity of a material is to be determined, acute or chronic adverse reactions to it, as well as its dose-response relationship, have to be checked by using biological tests. A toxicity test provides the crucial baseline data, and it is essential to check the safety of test substances such as drugs [14]. The main purpose of implementing a toxicity test is to estimate the safety of a new medicine and to determine its clinical safety. There are three kinds of toxicity tests; an acute toxicity test (single-dose toxicity test), a subacute toxicity test (1-month repeated-dose toxicity test), and a chronic toxicity test (dose toxicity test repeated over three months) [12].
Commonly, a chemical substance is not toxic if its concentration and/or exposure time in soft tissues are/is low enough. Toxic action by a specific chemical substance (toxic substance) can show various results of a chemicophysical character, depending on the exposure route and the susceptibility of the biological system or human body, so the toxicity has to be measured in many ways. Major factors affecting the toxicity of a specific chemical substance are the route, time, and frequency of administration. The route of administration is the most effective factor in the following order: intra-venous > inhalation > intra-peritonealperitoneal > subcutaneous > intra-muscular > endothelium > oral supplement > intralesional [12].
A testing protocol and acute, subacute or special toxicity test guidelines have been widely used in South Korea for a long time to identify unwanted toxic components in a chemical substance [4].
At the Ministry of Food and Drug Safety, toxicity test criteria are rigidly enforced, and all tests will be recognized if they are conducted according to the GLP regulation. When registering requests for permission to use a medicine, the following data have to be submitted to determine the stability and the effectiveness of that medicine: origin and process of discovery (data on mixing and use included), chemicophysical properties, standard drug stability data, and toxicity data. In the case of a preserved medicine and tar color, is the toxicity data are based upon the data for the new medicine, the data from single-dose, repeated-dose toxicity tests data, and other necessary toxicity test data [14]. Recently, the controversy over the stability of Korean medicine has been increasing, so single-dose and repeated- dose toxicity tests to determine the effectiveness of Korean medicines are necessary to verify the safety of Korean medicines to developing new, effective, safe herbal medicines [11].
This study addressed the toxicity and the lethal dose of neutral natured blood stasis pharmacopuncture administered by single-dose intramuscular injection in Sprague-Dawley 6-week-old male and female rats. Experimental groups were classified according to the administered dose of extract: 0.1, 0.5, and 1.0 mL/animal, respectively. The control group received an injection of normal saline. Single doses were administered via IM injection in 5 male and 5 female rats in each group. For the 14 days following injection, general symptoms and body weights were observed. After the observation period, hematologic and blood biochemical tests, necropsy, and local resistance tests were performed in the injected region.
No deaths occurred in any of the groups. Also, the injected extract appeared to have no influence on the general symptoms, body weights, hematologic and blood biochemical tests, and necropsy. Local resistance tests in the injected regions showed no influence due to the injected extract. Under the conditions of this test, for intramuscular injection of the extract neutral natured blood stasis pharmacopuncture in rats, the approximate lethal dose is estimated to higher than 1.0 mL/animal in both male and female rats.
The objective of this study was to analyze the single-dose toxicity of neutral natured blood stasis pharmacopuncture extracts. All experiments were conducted under the regulations of GLP at Biotoxtech, an institution authorized to perform non-clinical studies. The results showed that administration of 1.0 mL/animal of neutral natured blood stasis pharmacopuncture extracts did not cause any changes in weight and did not result in any mortalities, which indicates that neutral natured blood stasis pharmacopuncture can be used as a safe treatment.