The fibrinolytic enzyme in snake venom is defined as an enzyme that can hydrolyze fibrin to liquidize insoluble fibrin-rich clots but not form fibrin clots from fibrinogen [1]. The fibrinolytic enzymes are classified as serine protease and metalloprotease based on the structure of the active site and in
The fibrin(ogen)olytic enzyme from snake venom has received attention as a medical agent to treat obstructive thrombosis and acute stroke by removing fibrinogen and fibrin from the blood. Thus, many kinds of the enzymes have been isolated and reported [5]. However, the enzyme for clinical application should not cause hemorrhage [1]. Fibrolase from
Choi [8] detected fibrinolysis activities in the chromatographic fractions containing polypeptides of 59kDa, 54kDa, 46kDa, 32kDa, 18kDa and 15kDA isolated from the snake venom of
2.1. Isolation of fibrinolytic enzyme from snake venom
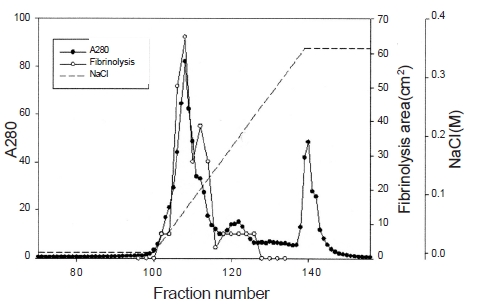
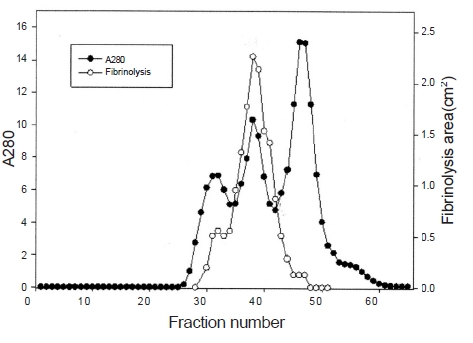
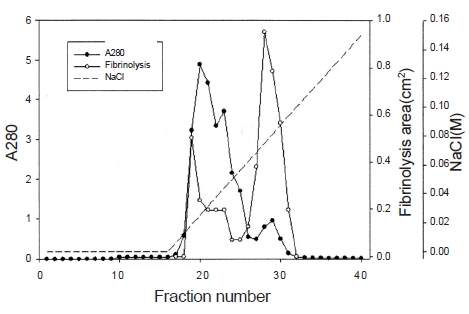
Ten g of lyophilized venom of
2.2. Sodium dodecyl sulfate-polyacrylamide gel elec-
trophoresis
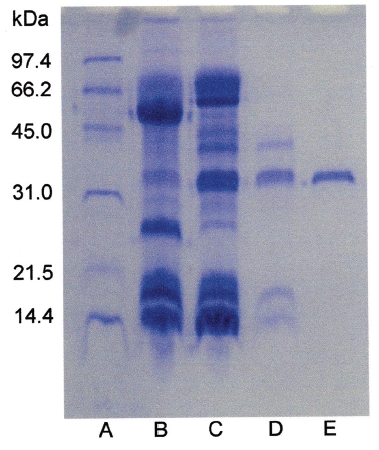
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the procedure described by Laemmli [12]. The molecular weight markers (Bio-Rad, USA) for SDS-PAGE were phosphorylase b (97.4kDa), serum albumin (66.2kDa), ovalbumin (45.0kDa), carbonic anhydrase (31.0kDa), trypsin inhibitor (21.5kDa), and lysozyme (14.4kDa). The sample buffer containing 2-mercaptoethanol was added to the sample and heated at 100℃ for 5 minutes.
2.3. Protein concentration determination
The protein concentration was determined using BCA Protein Assay Reagent (Pierce, USA). Bovine serum albumin was used for calibration line.
2.4. Protein hydrolysis analysis
Fibrinogen and gelatin were hydrolyzed using the isolated fibrinolytic enzyme and then subjected to SDS-PAGE. An aliquot (200 μl) of 10 mg/mL protein in 50 mM Tris- HCl pH 7.6, 0.15 M NaCl was mixed with 50 μl of 2 μg/mL fibrinolytic enzyme. The mixture was incubated at 37℃ for 6 hours. The samples (10 μl) which were taken from the mixture at 0.5, 1, 2, 4, and 6 hours were added to the sample buffer of SDS-PAGE, respectively. The mixture was heated at 100℃ for 5 minutes before SDS-PAGE.
The experiment to examine the effect of the isolated fibrinolytic enzyme on hemorrhage reaction beneath the back skins of mice was approved by the Animal Experiment Ethics Committee of Sangji University (Approval document No. 2102-9). An aliquot (0.1 mL) of fibrinolytic enzyme in 50 mM Tris-HCl, pH 7.6, 0.15 M NaCl at an appropriate concentration was injected subcutaneously into the back skin 6-week-old Institute of Cancer Research (ICR) mice of 6 week old (Daehan Biolink, Korea). The mice were sacrificed using cervical dislocation after 6 hr and the skin around the injection was stripped to determine the diameter of the hemorrhage zone beneath the skin. Two diameters (r1 and r2) at a right angle were measured. The hemorrhage area was calculated using the formula of 0.785r1r2. The hemorrhage areas were measured in duplicate by injecting the fibrinolytic enzyme into two mice, and the average was calculated.
2.6. Amino acid composition analysis
The amino acid composition of the fibrinolytic enzyme was analyzed at the Korea Basic Science Institute. The PITC-labeled amino acids were analyzed using a Pico-Tag Column and the HPLC instrument which consisted of a 510 HPLC pump, a gradient controller, and a 2487 UV detector (Waters Corporation, USA).
The fibrinolytic enzyme with a molecular weight of 32kDa (FE-32kDa) described by Choi [8] was obtained from
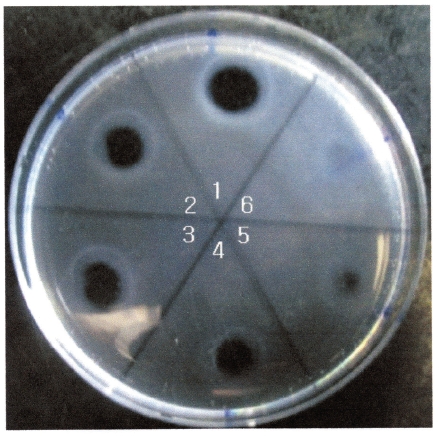
The areas of the fibrinolytic zones in the fibrin plate assay were 0.79 cm2, 0.64 cm2, 0.50 cm2, 0.38 cm2 at 0.5 mg/ mL, 0.4 mg/mL, 0.3 mg/mL, and 0.2 mg/mL of FE-32kDa, respectively (1-4 in Fig. 5). The fibrin plate assay showed that the minimum concentration of FE-32kDa to form a fibrinolytic zone was ten times lower than that of FE-27kDa and similar with that of FE-54kDa [8, 9].
Protease inhibitors were added to the solution containing FE-32kDa (0.5 mg/mL) in the fibrin plate assay. The relative fibrinolysis was the ratio of the area of the enzyme treated with protease inhibitor to that of a control without treatment (Table 1). Table 1 shows that all of the chelate compounds, including EDTA, EGTA, 1, 10-phenanthroline, inhibited fibrinolysis completely. PMSF, a serine protease inhibitor, inhibited fibrinolysis partially and
Dithiothreitol and cysteine, which cleave disulfide bonds, inhibited fibrinolysis completely. However, iodoacetate, which alkylates sulfhydryl groups, did not show any effect. These results suggest that FE-32kDa is a metalloprotease which has disulfide bonds necessary for the fibrinolytic activity.
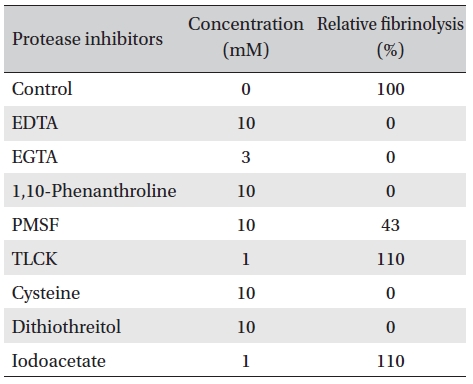
Effects of protease inhibitors on the fibrinolytic activity of FE-32kDa from G. b. siniticus venom
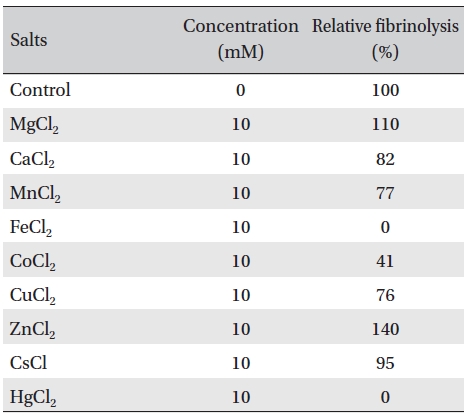
The effects of metal ions on the fibrinolytic activity of FE- 32kDa were determined (Table 2). The concentration of metal ions added to FE-32kDa (0.5mg/mL) was 10 mM. Table 2 shows that Fe2+ and Hg2+ inhibited the fibrinolytic activity completely and that Co2+ inhibited it strongly. Ca2+, Mn2+, and Cu2+ inhibited the fibirinolytic activity weakly and Mg2+ and Cs2+ had little effects on the fibrinolytic activity. However, Zn2+ increased the fibrinolytic activity by up to 40%. These results suggest that FE-32kDa is a metalloproteinase which requires Zn2+ at the active site of the enzyme. The zinc ion at the active site of the enzyme seems to have been partially lost during the purification procedures of the enzyme, and subsequent addition of Zn2+ in the fibrin plate assay seems to have increased the enzymatic activity.
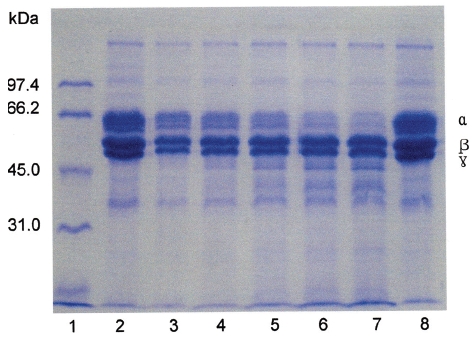
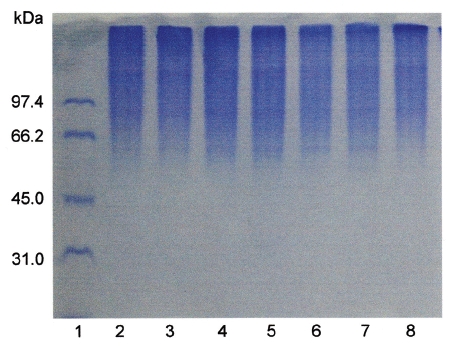
Fibrinogen (16 mg/mL) was mixed with FE-32kDa (1.6μg/mL) and incubated at 37℃ for a certain time. The sample was subjected to SDS-PAGE to determine the fibrinogen hydrolysis pattern of FE-32kDa (Fig. 6). The SDS-PAGE pattern showed that the intensity of α-chain of fibrinogen decreased continuously throughout the incubation and was very low after 4 hours (Fig. 6, lane 6, 7). However, no change was noted for
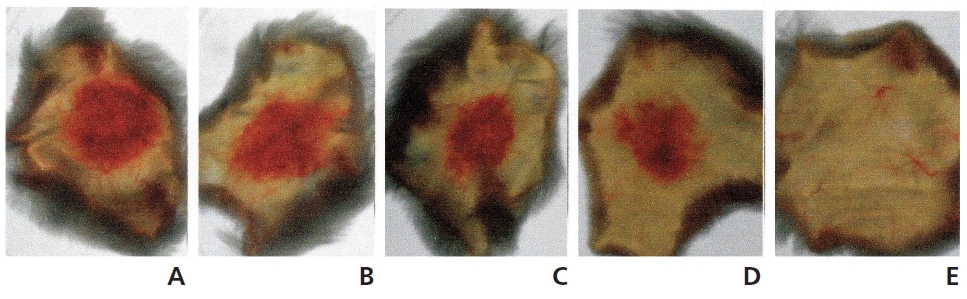
The hemorrhagic activity of FE-32kDa was determined by injecting 100 μl of FE-32kDa at various concentrations subcutaneously into the shaved back skin of mice. The mice were sacrificed after six hour and then the area of the hemorrhage zone (Fig. 8) formed beneath the skin was determined. When FE-32kDa was administered to a mouse at the dosages of 20 μg, 10 μg, 5 μg, and 2 μg, the areas of the hemorrhage zone were 1.8 cm2, 2.2 cm2, 1.4 cm2, and 1.0 cm2, respectively (A-D in Fig. 8) The hemorrhagic activity of FE-32kDa at the low dosage suggests that the enzyme is not appropriate for clinical treatment of obstructive thrombosis and acute stroke.
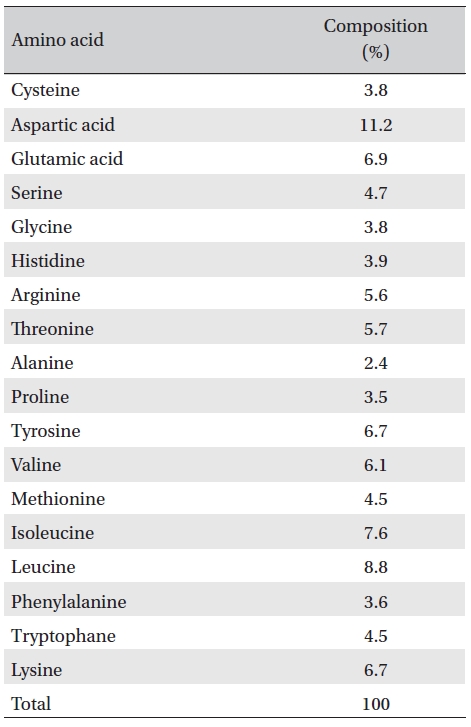
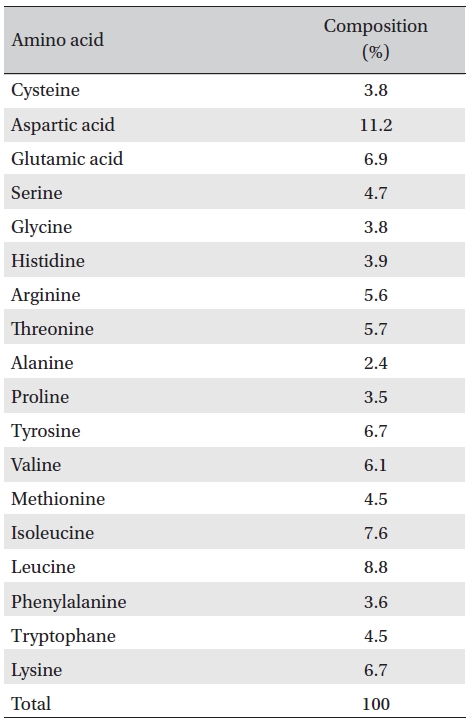
The amino acid composition of FE-32kDa is shown in Table 3. The enzyme contains cysteine residues which may be relevant to inactivation of the enzyme by cysteine and dithiothreitol (Table 2). The contents of aspartic acid and leucine which are the major amino acid residues in FE- 32kDa are 11.2% and 8.8%, respectively (Table 3).
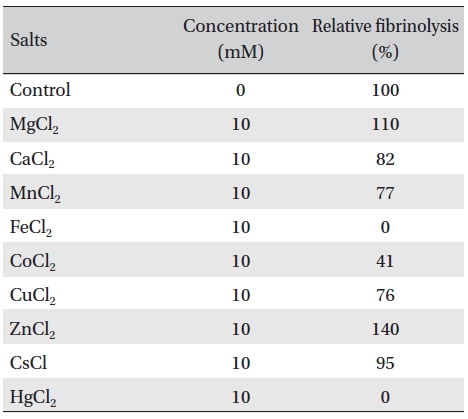
Effects of metal salts on the fibrinolytic activity of FE- 32kDa from G. b. siniticus venom.
Three fibrinolytic enzymes, FE-54kDa [8], FE-27kDa [9], and FE-32kDa, were isolated from the venom of
Brevilysin H1 from
Terada and his colleagues studied the metalloproteases, such as brevilysin H6 [14, 15], brevilysin L4 [16, 17] and brevilysin L6 [18], isolated from the venom of Chinese mamushi,
The molecular weights of brevilysin L4 and L6 were 22kDa and 21.5kDa, respectively [16, 17]. Hydrolysis of fibrinogen by brevilysin L6 was slower than brevilysin L4. The complete amino acid sequence of brevilysin L4 indicats that the enzyme is a PI class metalloprotease [18]. The nucleotide sequence of a cDNA clone encoding brevilysin L4 has revealed that the gene of brevilysin L4 encodes a PII-class metalloprotease, which undergo post-translational autoproteolysis to split into brevilysin L4 and disintegrin.
[Table. 3] Amino acid composition of FE-32kDa.
Amino acid composition of FE-32kDa.
The molecular weight of FE-32kDa suggests that the enzyme should belong to the PII class, which may also be split into a PI-class metalloprotease similar with non-hemorrhagic brevilysin L4. Further studies are required to isolate a non-hemorrhagic brevilysin L4-like fibrinolytic enzyme from