In homeopathy, it is claimed that more homeopathically-diluted potencies render more protective/curative effects against any disease condition. Potentized forms of Condurango are used successfully to treat digestive problems, as well as esophageal and stomach cancers. However, the comparative efficacies of Condurango 6C and 30C, one diluted below and one above Avogadro’s limit (lacking original drug molecule), respectively, have not been critically analyzed for their cell-killing (apoptosis) efficacy against lung cancer cells in vitro, and signalling cascades have not been studied. Hence, the present study was undertaken.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenylte-trazolium bromide (MTT) assays were conducted on H460-non-small-cell lung cancer (NSCLC) cells by using a succussed ethyl alcohol vehicle (placebo) as a control. Studies on cellular morphology, cell cycle regulation, generation of reactive oxygen species (ROS), changes in mitochondrial membrane potential (MMP), and DNA-damage were made, and expressions of related signaling markers were studied. The observations were done in a “blinded” manner.
Both Condurango 6C and 30C induced apoptosis via cell cycle arrest at subG0/G1 and altered expressions of certain apoptotic markers significantly in H460 cells. The drugs induced oxidative stress through ROS elevation and MMP depolarization at 18-24 hours. These events presumably activated a caspase-3-mediated signalling cascade, as evidenced by reverse transcriptase- polymerase chain reaction (RT-PCR), western blot and immunofluorescence studies at a late phase (48 hours) in which cells were pushed towards apoptosis.
Condurango 30C had greater apoptotic effect than Condurango 6C as claimed in the homeopathic doctrine.
In view of growing awareness of the side-effects of some orthodox medicines, people are turning more towards alternative medicines with fewer and less-toxic side-effects [1], and homeopathy has become a major complementary and alternative medicine (CAM) in many countries today. In homeopathy, microdoses of very high dilutions (potentized) of natural substances are generally preferred over mother tinctures (crude extracts) [2-4] for stronger and longer-effects. The initial drug substance is generally dissolved in an aqueous solution of ethanol (mostly 70%) and is potentized in gradual steps of dilution with agitation or succussion. On a centesimal scale, when 1 mL of mother tincture is diluted with 99 mL of an aqueous solution of ethanol (vehicle of drug) and given 10 mechanical jerks, potency 1C is produced. When 1 mL of 1C is again diluted with 99 mL of an aqueous solution of ethanol and given 10 jerks, the potency 2C is produced, and so on. Therefore, when the drug has attained potency 12C, it has been diluted to 10-24 (beyond Avogadro’s limit), and the existence of even a single molecule of the original drug substance becomes highly improbable. Although some researchers have demonstrated the existence of nanoparticles of the original drug in such ultra-highly diluted homeopathic drugs [5, 6], the efficacy is often questioned by rationalists, as the precise mechanism of drug action has still not been firmly established. Therefore, we became interested in the study of any perceivable differences in the actions between dilutions below Avogadro’s limit (6C, diluted 1012 times) and above Avogadro’s limit (30C, diluted 1060 times) in living cells,
Lung cancer is the leading cause of cancer deaths worldwide [7]. Non-small cell lung cancer (NSCLC) is the most prevalent accounting for - 80% of all lung cancer cases. In addition, benzo[a]pyrene is one of the major polycyclic aromatic hydrocarbons found in cigarette smoke and is responsible for inducing lung tumours in smokers [8]. Patients with lung cancer are commonly treated with conventional modalities including chemotherapy, radiation therapy, etc. which also affect normal cells. Thus, CAMs, including homeopathy, acupuncture, etc., are now gaining importance in the cure/amelioration of many difficult- to-cure diseases including cancer.
The present investigation, therefore addressed two questions. First, do the two potentized homeopathic drugs, Condurango 6C and 30C show any ability to induce apoptosis in NSCLC, and do they act via reactive oxygen species (ROS) generation and mitochondrial membrane potential (MMP)-depolarization. Second, dose if Condurango 30C have more apoptosis-inducing ability than Condurango 6C.
NCI-H460 (H460) human NSCLC cells were procured from National Centre for Cell Science (NCCS), Pune, India and cultured in RPMI-1640 media, supplemented with 10%-fetal bovine serum (FBS) and 1%-antibiotic-antimicotic solution. The homeopathic potentized drugs Condurango 6C and 30C were procured from Schwabe India Pvt. Ltd., Kolkata.
H460 cells (1×104/well) were treated with different concentrations of Condurango 6C and 30C (0.5μL/100μL media- 5μL/100μL media) and with placebos (successed 70% alcohol - vehicle for the drugs) as a control for 24 hours and 48 hours. The concentrations at which both the drugs showed nearly 50% cell death were determined by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays [9].
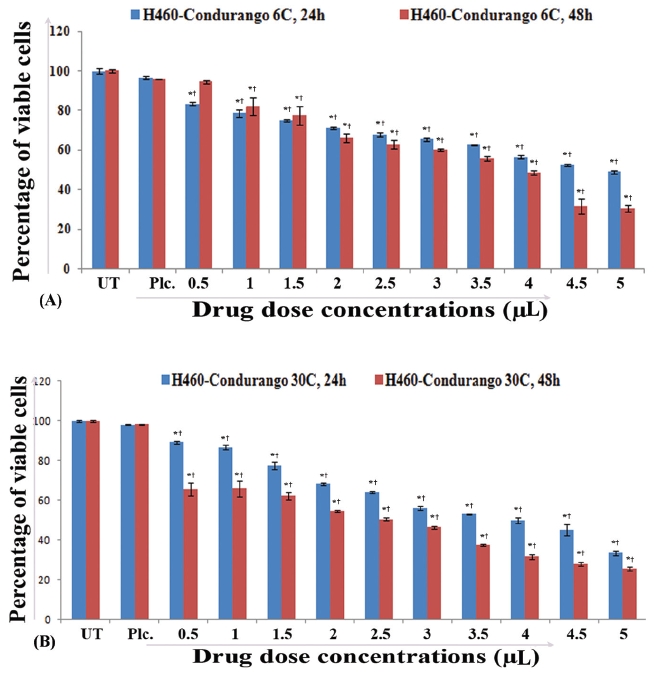
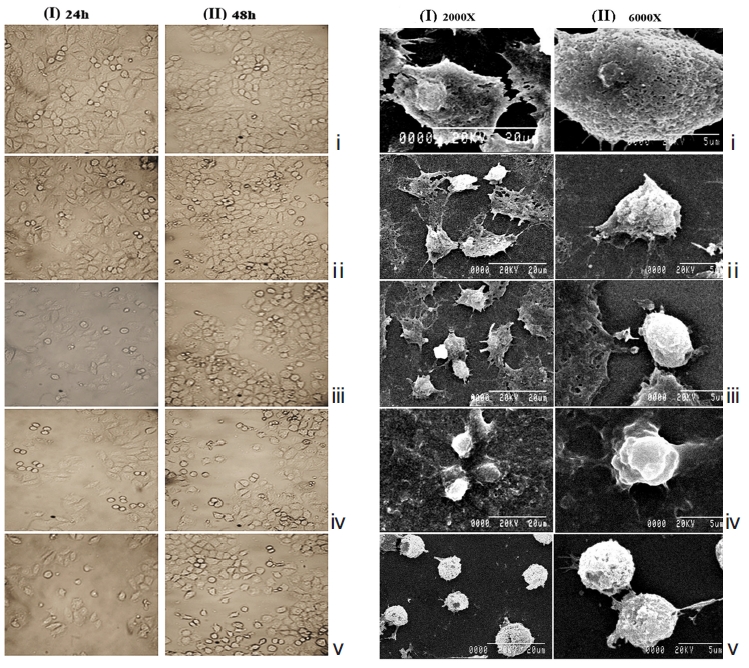
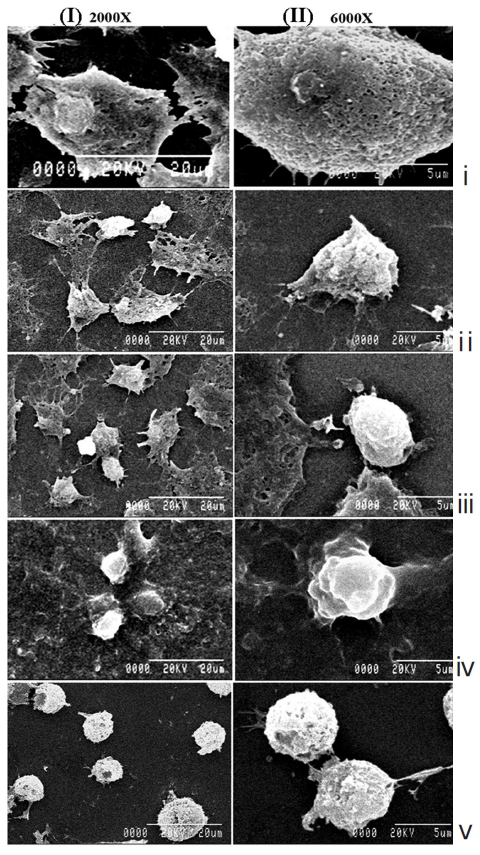
H460 cells were also treated with half maximal inhibitory concentration (IC50) doses of Condurango 6C and 30C and were compared against placebo-treated and untreated cells. After 24 hours and 48 hours of treatment, the cells were observed and photographed under an inverted phase-contrast microscope (Axiscope plus 2, Zeiss, Germany). For further confirmation of morphological changes, if any, a scanning electron microscopy (SEM) study was done [10] using an S530-Hitachi scanning electron microscope.
Reactive oxygen species (ROS)-accumulation is generally known to occur at early hours of apoptosis [11]. Cells were treated with Condurango 6C and 30C (IC50 doses) and with their respective placebos for 2 hours , 6 hours, 12 hours, 18 hours, and 24 hours, to estimate the specific time-point at which maximum ROS accumulated. After treatment, cells were incubated with 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA) (5mM) and ROS was estimated by using fluorimetry (PerkinElmer, USA). Fluorescence microscopy was done at the specific time-point(s) when ROS-generation was maximum.
The changes in MMP were recorded at 2 hours , 6 hours, 12 hours, 18 hours, and 24 hours of treatment with IC50 doses of both drugs were compared against drug-untreated cells by using Rhodamine-123 and the cells were analyzed by using fluorescence microscope (Leica, DMLS) [12]. Changes in MMP were also measured using flowcytometry (FACS, Aria III, BD Bioscience) at the specific time-point(s) at which the MMP showed the maximum decrease.
Cells were stained separately with 4’,6-diamidino-2-phenylindole (DAPI) (10μgmL-1) and Acridine Orange/Ethidium Bromide (AO/EB) (1mg/mL) to visualize changes in nuclear morphology [13]. After 48 hours of treatment, stained cells were observed and photographed under a fluorescence microscope (Leica, DMLS).
DNA-fragmentation was assayed using the conventional phenol/chloroform method [14] and was visualized under a UV-transilluminator (Ultracam Digital Imaging, Genei, India).
DNA strand breakage was analysed flowcytometrically by labeling the treated and the untreated cells with 5-Bromo- 2´-Deoxyuridine 5´-Triphosphate (Br-dUTP) [15] by using BD FACS VerseTM.
Fixed cells were incubated with propidium iodide (50μg/ mL) [16]. The percent of cells in each phase (subG0/G1, G0/G1, S, and G2/M) was quantified flowcytometrically using BD FACS VerseTM.
RT-PCR analysis was done using primers of Bax, Bcl2, cytochrome-c, caspase-3 and GAPDH [17]. The primer sequences are mentioned in Table 1. Band intensities were measured densitometrically using ‘image J’ software.
[Table. 1] Primer sequences of the respective genes
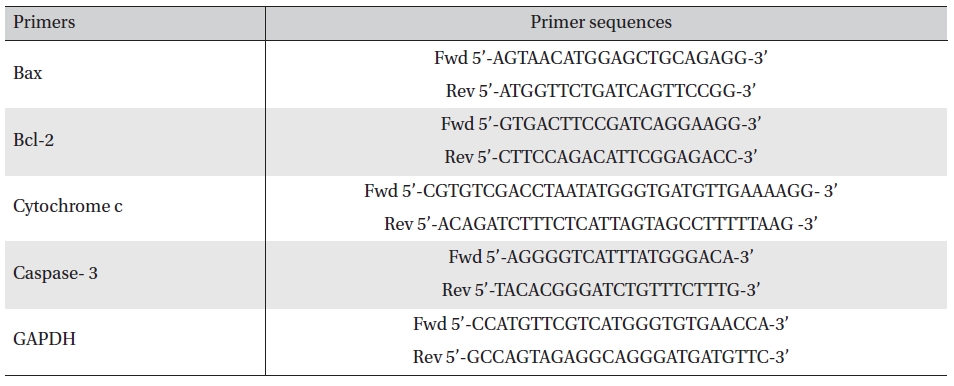
Primer sequences of the respective genes
The cells were treated with IC50 dose of both drugs for 18 hours in the case of Bax-Bcl2, 24 hours for cytochrome-c, and 48 hours for caspase-3 and poly (ADP-ribose) polymerase (PARP). The protein activities were measured by using indirect- enzyme linked immunosorbent assay (ELISA) and western blot analyses [18]. Quantifications of developed proteins after the western blot analyses were done densitometrically by using image J software.
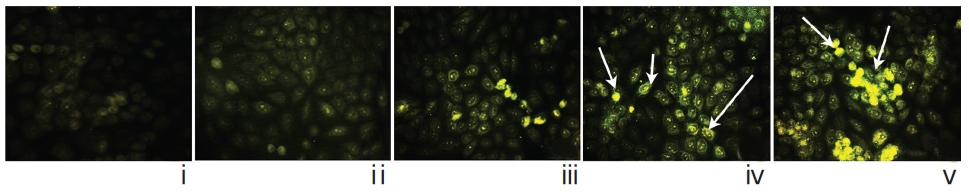
Localization of caspase-3 was done by using an immunofluorescence study [19] and was photographed under fluorescence microscope.
The observers were blinded during observation as to whether they were observing the control and/or drug-treated materials.
Data were analyzed, and the significance of differences between the mean values was determined by using a one-way analysis of variance (ANOVA) with Fisher’s least significant difference (LSD) post hoc tests by using SPSS 14-software (SPSS Inc, Chicago, IL, USA). Statistical significance was considered at
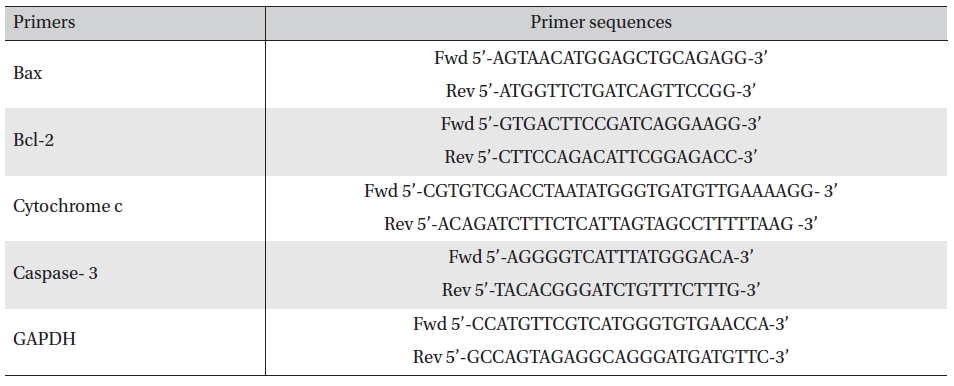
IC50 values for 48 hours treatment with Condurango 6C (3.57μL/100μL) and Condurango 30C (2.43μL/100μL) against H460 were selected for the entire study (Fig 1) The cell viability of both placebo-treated cells at maximum dose (5μL/100μL) was found to be very close to that of the untreated (control) ones.
The light microscopy (Fig. 2) and the SEM (Fig. 3) studies revealed that cell morphologies remained unaltered with intact cell membrane and cellular extensions in both untreated and placebo-treated cells. However, Condurango 6C-treated cells showed gradual deformation with cellular shrinkage; Condurango 30C-treated cells became gradually smaller and rounded with distorted nuclei and damaged cellular extensions, depicting the great apoptosis-inducing potential of Condurango 30C.
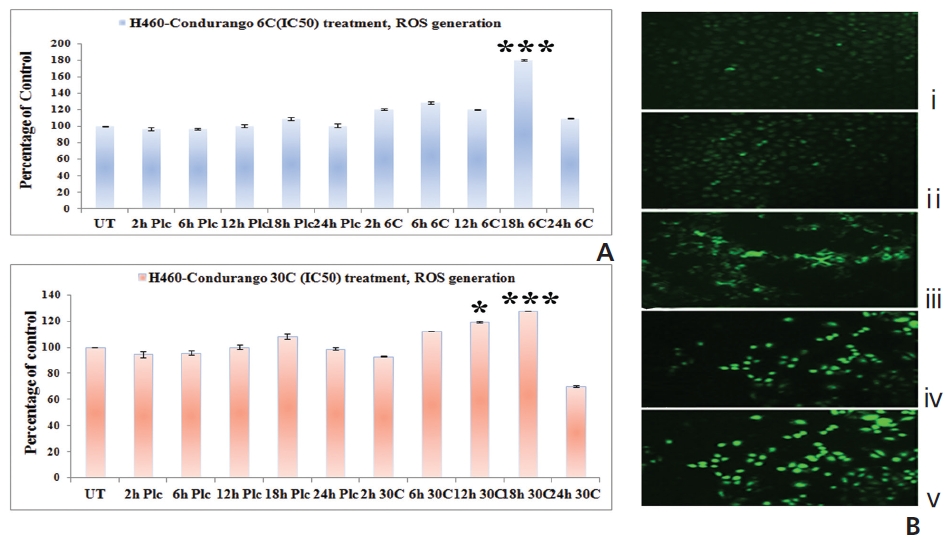
The fluorescence intensity in optical density (OD) was too low in untreated and placebo-treated cells at different hour intervals. A significant increase in the fluorescence intensity was observed at 18 hours in both Condurango 6C and 30C-treated cells (Fig. 4A). Fluorescence microscopy showed a greater H2DCFDA-intensity in drug-treated cells at 18 h (Fig. 4B) than in drug-untreated cells.
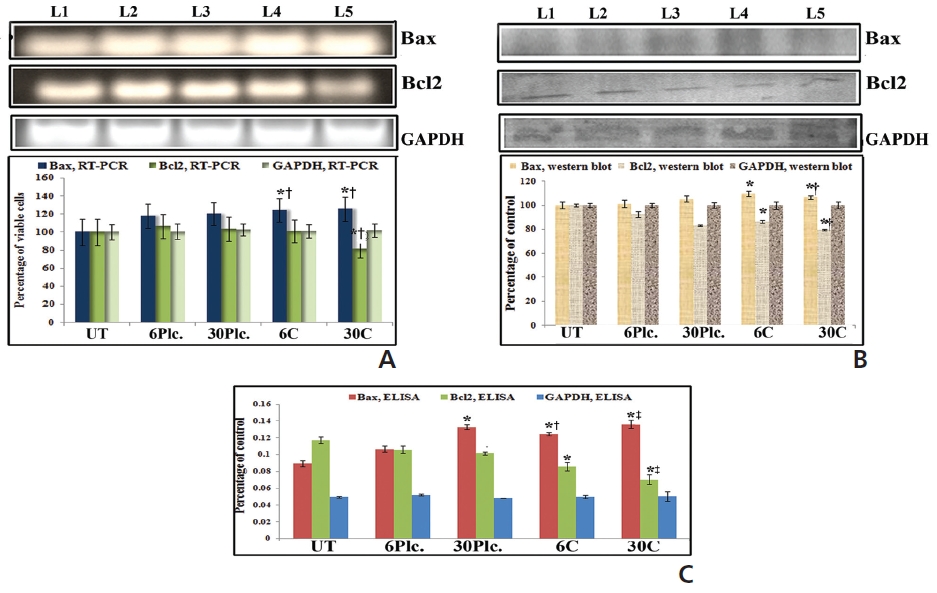
Results showed up-regulation and downregulation of Bax and Bcl2, respectively, at 18 hours in response to both drug treatments as compared to drug-untreated cells (Fig. 5). Condurango 30C-treated sample showed significantly increased Bax and decreased Bcl2-expressions than Condurango 6C and placebo-treated samples.
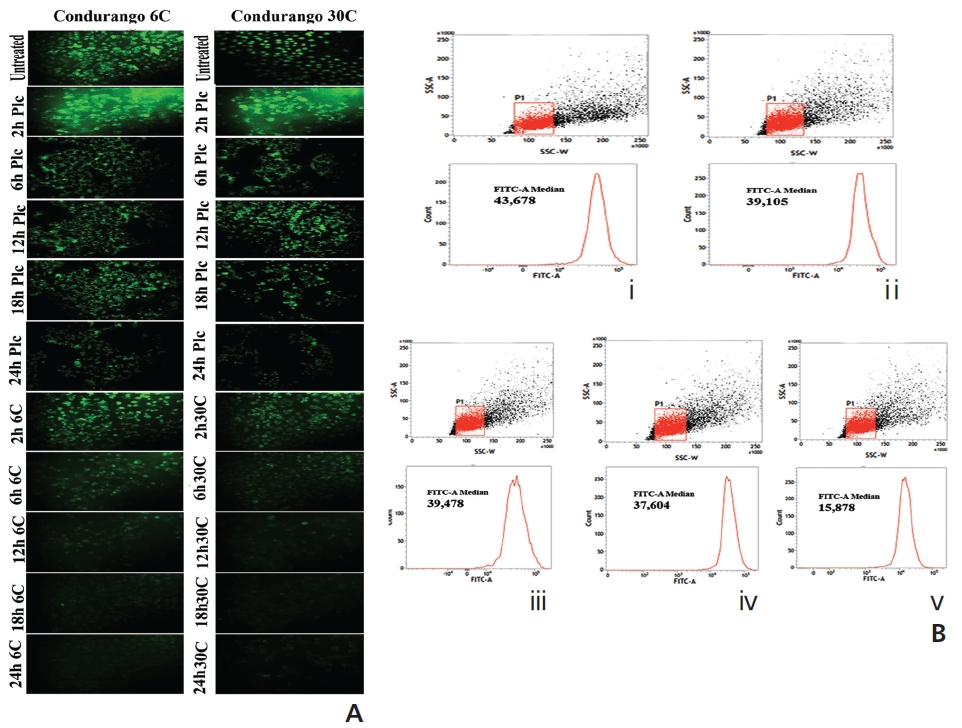
Figure 6A shows bright green fluorescence in drug-un-treated and cells at different time-points while Condurango 6C and 30C-treated cells showed a gradual decrease in the fluorescence intensity with increasing time, but a marked decrease occurred at 24 hours, suggesting that MMP depolarizes the maximum at 24h that trigger cytochrome- c release from mitochondria. The flowcytometric analysis also supported the data (Fig. 6B).
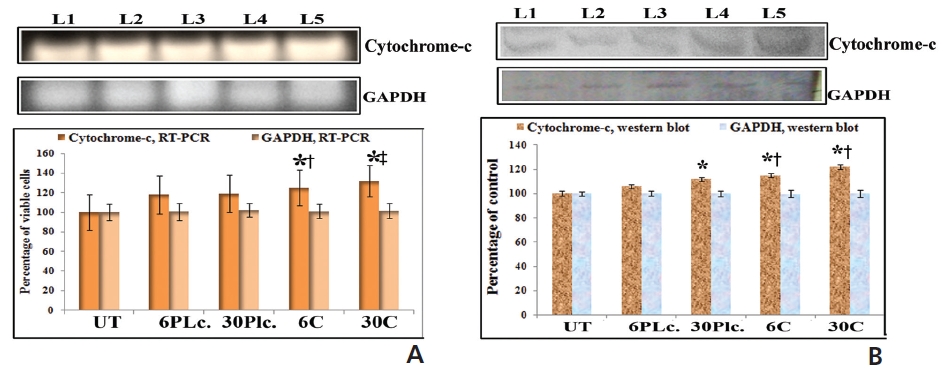
An increased expression of cytochrome-c was found in response to drugs at 24 hour of treatment (Fig. 7). Condurango 30C-treated cells showed more significant upregulation of cytochrome-c than Condurango 6C-treated ones.
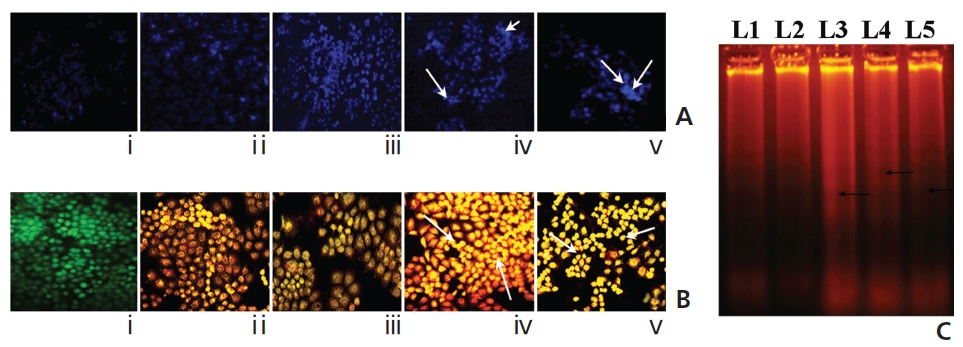
A gradual increase in the DAPI-fluorescence intensity was observed in Condurango 6C and 30C-treated cells at 48 hours of treatment (Fig. 8A). Further, AO/EB- staining showed both a change in the fluorescence pattern from green (normal-cellular DNA) to orange (nicked-cellular DNA) and an increase in the fluorescence of EB in drug-treated cells (Fig. 8B). Figure 8C shows the formation of DNA-laddering in both drug-treated samples, especially in Condurango 30C-treated samples, compared to the DNA in the drug-untreated groups.
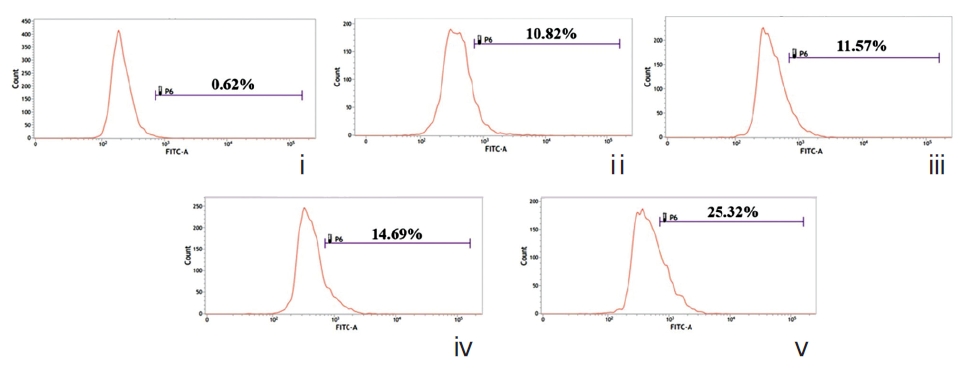
A gradual increase in the number of dUTP-nicks was noted in drug-treated cells compared to drug-untreated cells (Fig. 9). This strongly suggests the abilities of both drugs to generate DNA-nicks that induce apoptosis. Quantitative evaluation revealed the percent of TUNEL-positive nuclei was greater in Condurango 30C-treated cells.
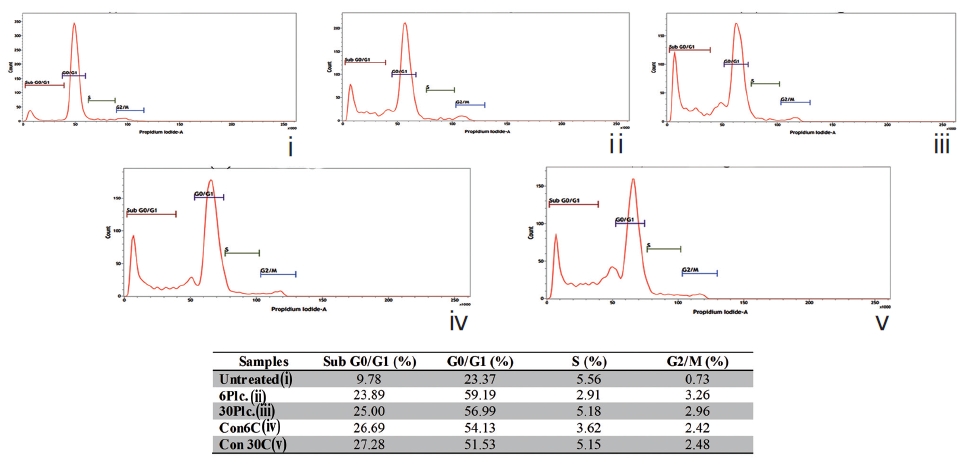
The sub-diploid cell population at subG0/G1 was more highly increased in the Condurango 6C and 30C-treated cells than in the placebo-treated and untreated cells (Fig. 10).
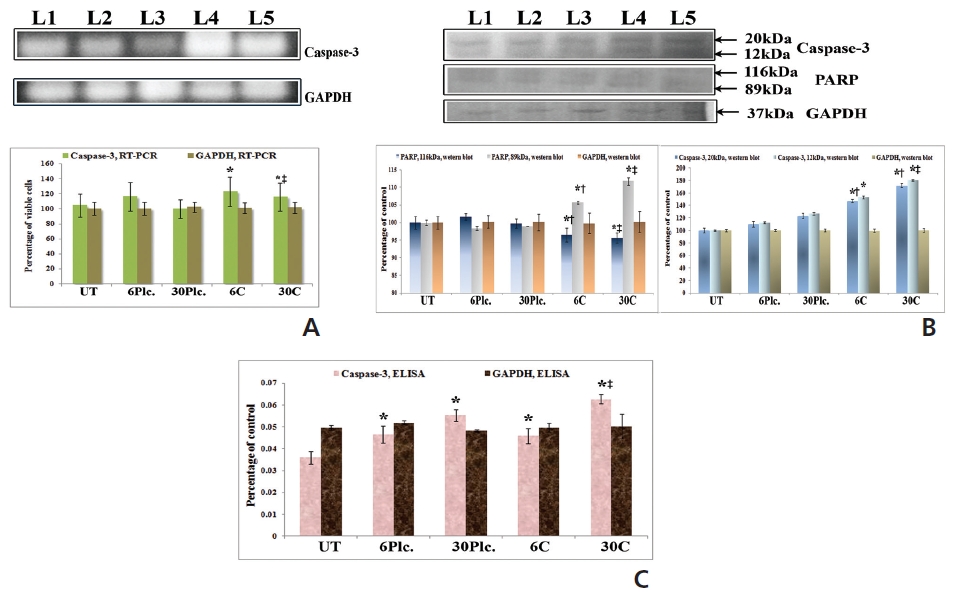
RT-PCR and ELISA data showed upregulation of caspase-3 after Condurango 6C and 30C treatment as compared to untreated samples (Fig. 11). The Western blot analysis revealed that Condurango 30C had more capacity to increase caspase-3 expression significantly by forming cleaved fragments (20kDa and 12kDa).
The immunofluorescence study demonstrated a gradual increase in the localization of caspase-3 within cell-cytosol in drug-treated cells, especially in Condurango 30C-treated cells (Fig. 12).
PARP activation, the end process of the caspase-3-mediated pathway is designated by the formation of two cleaved fragments of 116kDa (inactive) and 89kDa (active). The Western blot analysis showed the formation of two fragments in Condurango 6C and 30C-treated cells at 48 h of treatment, which was nearly absent in drug-untreated samples (Fig. 11B). Band-intensities confirmed gradual down-regulation of the 116kDa fragment and significant up-regulation of the 89kDa fragment, especially in the Condurango 30C-treated cells.
Present findings would demonstrate that exposure of NSCLC- H460 cells to IC50 doses of both Condurango 6C and 30C for 48 hours resulted in apoptotic cell-death. Further, the effects of Condurango 30C, which actually was highly- diluted, caused relatively more palpable alterations in all parameters of this study than did Condurango 6C. This phenomenon did not apparently follow the general pharmacological rule that the effect of a drug increases linearly with its concentration, but was in line with the claim of “the higher the dilution, the stronger the effect” as per homeopathic doctrine. Incidentally, apparent evidence for discernible effects produced by two dilutions, one below and one above Avogadro’s limit an enigma to many rationalists who believe in the accepted laws of physical sciences, as well as pharmacological sciences [20]. However, claims are accumulating that homeopathic potencies beyond Avogadro’s limit show demonstrable beneficial/ curative effects against different diseases including cancer [20-22]. In fact, our earlier
Apoptosis induction in cancerous cells is often targeted as one of the key events of cancer chemotherapy. Thus, one approach was to look at the generation of ROS and to ascertain if it had a specific role in the induction of apoptosis. The time-course studies showed elevation of ROS, MMP-depolarization and cytochrome-c release at 18 hours through 24 hours of treatment, indicating that these events were earlier than apoptotic execution at 48 hours. Our result for cytochrome-c release at 24 hours of treatment further suggested that these events occurred due to an alteration in the mitochondrial structure that could trigger apoptosis.
Along with mitochondrial dysfunctions, ROS generation may be initiated at early hours of drug exposure via Bax- Bcl2 modulation [11]. In this study, we found increased and decreased expression of Bax and Bcl2, respectively, at 18 hours in the drug-treated series. However, surprisingly Bax-Bcl2 expressions were more significant in the Condurango 30C-treated samples, which is a good indication of the higher efficacy of a more highly diluted homeopathic drug.
Another indication of apoptosis is internucleosomal DNA breakdown. Results of DAPI and AO/EB-staining would suggest the formation of DNA nicks, as further confirmed by DNA-laddering in drug-treated cells. TUNEL-positivity in both drug-treated cells as compared to placebo- treated cells would further strengthen ROS-dependent DNA-breakage-mediated apoptotic events. Interestingly quantitatively more DNA damage was found in Condurango 30C-treated cells, than was found in Condurango 6C.
Caspase-3 activation is one of the hallmarks of apoptosis- mediated cell death. Increased ratio of Bax-Bcl2 is well known to stimulate the release of cytochrome-c from mitochondria to promote activation of caspase-9 that binds to apaf-1 to lead to caspase-3 and PARP-activation [7]. Results provide clear indication of caspase-3 activation in drug-treated cells. Increased expression and formation of cleavage of caspase-3 and PARP after drug exposure confirmed apoptosis induction via caspase-3-mediated- intrinsic pathway. However, noticeably, Condurango 30C-treated cell showed more significant modulation of caspase-3 and PARP cleavage than did Condurango 6C-treated cells. These strongly support again the idea that more potentized Condurango 30C had greater ability to induce apoptosis in NSCLC-H460.
If the similar results are taken into consideration, one possible conclusion is that homeopathic drugs may act by interacting with certain high affinity receptors that regulate expressions of specific genes [23], but more work is necessary to identify some missing links. The present study has gain significance because it attempts to determine if potentized forms of Condurango, below (6C) and above (30C) Avogadro’s limit, which are occasionally used to treat digestive problems and stomach cancer [22, 24], had abilities to inhibit lung cancer progression,
The various changes noted in this study can only be brought about by the activities of certain genes and by epigenetic modifications, which would support the hypothesis [3, 23] that ultra-highly-diluted drugs might somehow manage to correct expressions of relevant genes, the regulation of which had failed in cancer cells. Interestingly, many factors, including the presence of nanoparticles [5, 25], interaction between containers (e.g., silica from glass or a polymer) and drug molecules, etc., are now under scrutiny and are implicated in modifications of the structural orientation, the size, and the physico-chemical properties of resultant homeopathic drugs even in absence of any original molecule [6, 26].
The overall results of this study suggest that Condurango 30C has more apoptosis-inducing ability than Condurango 6C, which is consistent with the claim made in the homeopathic doctrine.