Dinoflagellate species composition has changed around Jeju Island as well as in Korean waters due to global warming and climate changes. An investigation was conducted to monitor changes in planktonic dinoflagellates around Jeju Island from June 2006 to September 2009. A total of 86 species belonging to 14 families and 15 genera were identified, of which 34 species were newly recorded in Korean waters. Among the newly recorded species, >20 were confirmed as tropical species. Thus, the occurrence of such tropical dinoflagellates could be an indicator to monitor of environmental changes including global warming around Jeju Island and in Korean waters.
The marine ecosystem around Jeju Island has been changing from temperate to sub-tropical and/or tropical conditions over the last two decades. We have several clues for the change such as occurrence of tropical fish, invertebrates and macro-algae. Here we propose two possibilities to explain these changes: 1) the increase in seawater temperature around Jeju Island by the East China Sea is occurring due to atmospheric circulation (Yeh and Kim 2010) and 2) expansion of the Kuroshio Current by rising sea level caused by global warming (Pang et al. 1996). Increasing seawater temperature and expansion of the high salinity and high temperature Kuroshio Current could have affected the marine ecosystem around Jeju Island, which would result in tropical planktonic and benthic species adapting to survive around Jeju Island.
Dinoflagellates are a major taxon in the phytoplankton community and are comprised of various species in terms of habitat and nutrient uptake. These species contribute to primary production in oceans and include diverse tropical species reported in many waters worldwide (Dodge 1982). Dinoflagellates produce resting cysts as a survival strategy in unfavorable conditions (Anderson and Wall 1978). The evidence for an ecosystem change around Jeju Island includes increases in sea surface water temperature and a rising sea level, as well as some changes in macroalgae and fish, but little planktonic evidence is available indicating these. Dinoflagellates could potentially be used as an indicator species for the changing marine ecosystem. A total of 153 planktonic dinoflagellates were described from Korean waters so far (Shim et al. 1981, Han and Yoo 1983a, 1983b, Yoo and Lee 1986, Lee et al. 1993, Shim 1994). However, no more dinoflagellates were taxonomically described until this present study.
The objectives of study were to collect planktonic evidence for the marine environmental changes near Jeju Island and to confirm the occurrence of newly recorded and tropical dinoflagellates, which may have been introduced by the Kuroshio Current.
Monthly sampling was performed at 14 stations around the coastal waters of Jeju Island from June 2006 to September 2009 (Table1). Planktonic dinoflagellates were collected in a plankton net with 20 μm mesh and fixed in formaldehyde (final concentration of 0.4%). The dinoflagellate samples were washed with distilled water and then dehydrated in an alcohol series (30%, 50%, 70%, 90%, and 100%) to prepare slide specimens. One drop of mount media was added to a cover slide and mounted upside down on the slide where some dinoflagellates had dried after the alcohol series. Successfully prepared slides were selected for use in identification. The preparations for scanning electron microscopy followed a method described in Yoo and Lee (1986).
Light microscopy (Axioplan; Carl Zeiss, Oberkochen, Germany) and scanning electron microscopy (JSM-6700F; JEOL, Tokyo, Japan) were used to identify specimens, and several monographs were cited from different oceans such as the Indian Ocean (Taylor 1976), Japanese adjacent sea (Yamaji 1984), British Isles (Dodge 1982), Atlantic Ocean (Dodge 1985), and Kuroshio Current (Fujioka 1990).
The dinoflagellate classification system according to Shim (1994) was applied. The genera
[Table 1.] Latitude and longitude of the sampling stations
Latitude and longitude of the sampling stations
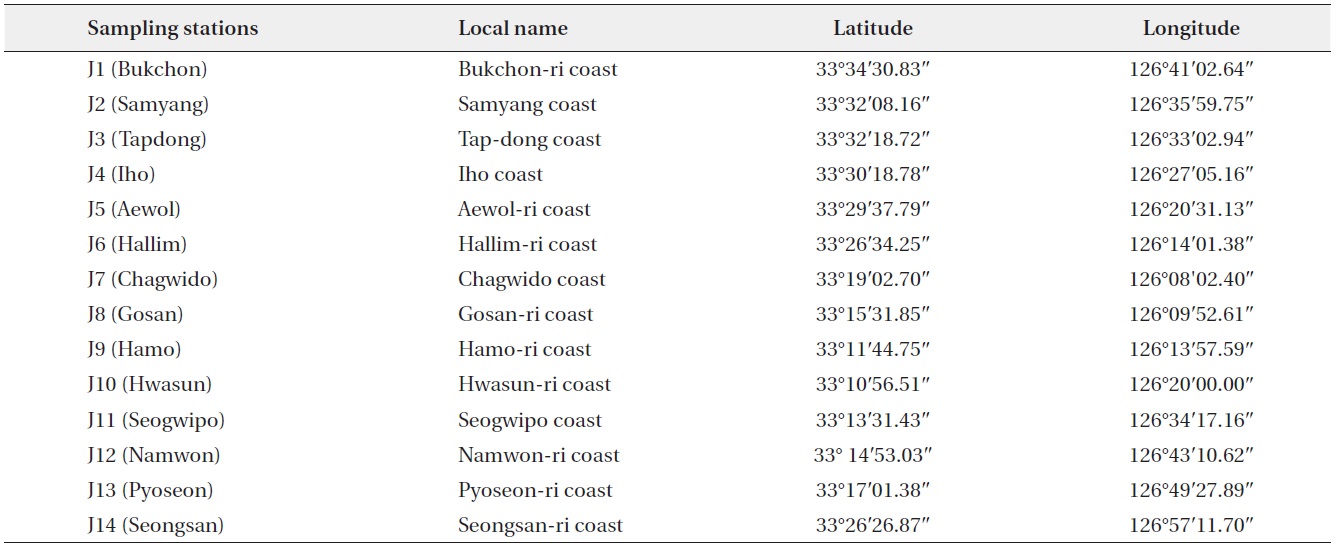
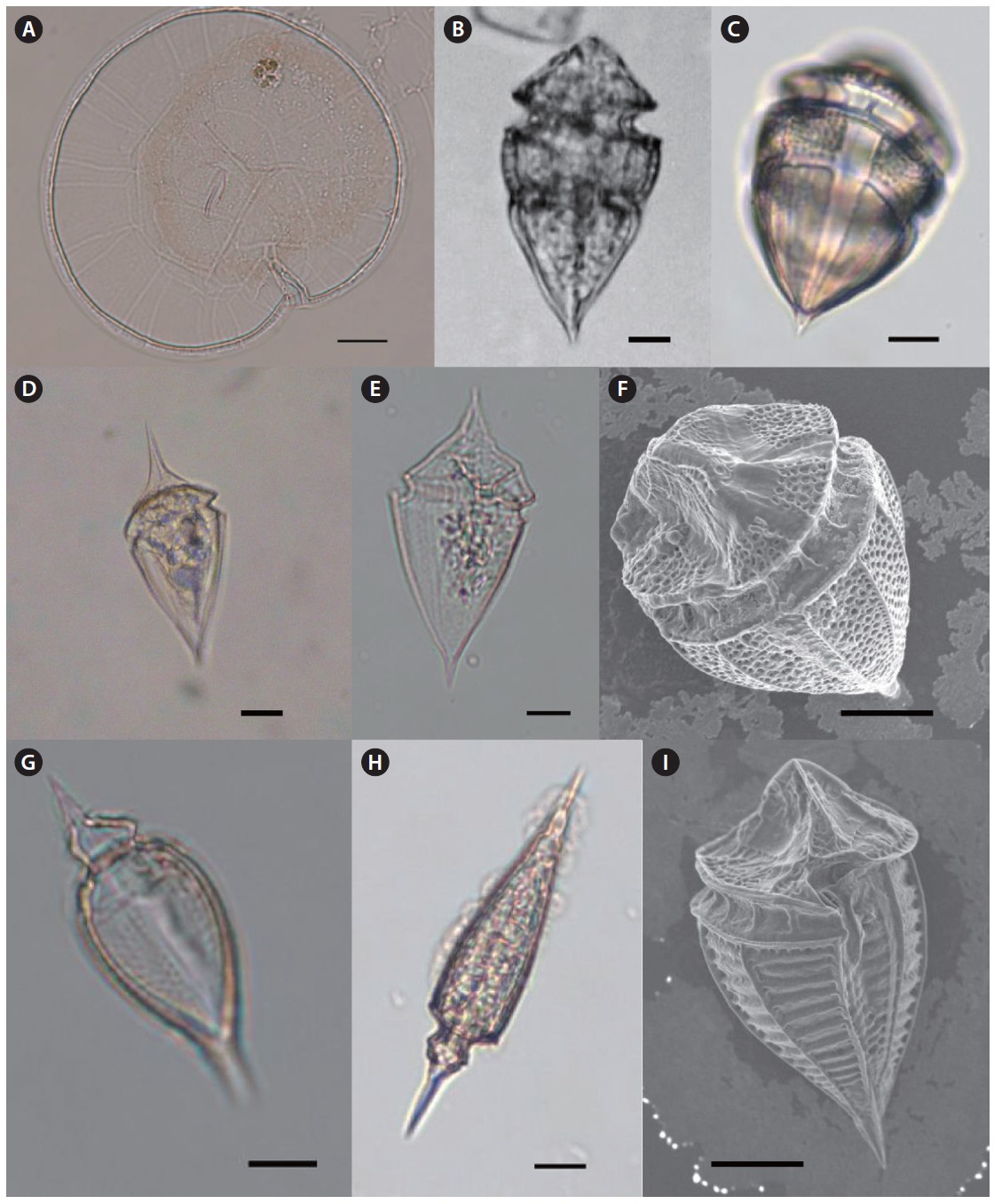
Eighty-six dinoflagellate species belonging to 7 orders, 14 families, and 15 genera were identified during the study period. Among them, 34 species were recorded for the first time in the sea adjacent to Jeju Island and in Korean waters. Of the unrecorded species, 23 were defined as tropical species and have been reported in tropical waters worldwide. The species that occurred are listed below and an asterisk (*) mark indicates a newly recorded species in Korean waters and newly described in this study. The species written in bold indicate tropical species among the newly recorded species in this study.
>
Systematics of Occurring Species
Class Dinophyceae
Order Prorocentrales Lemmermann
Family Prorocentraceae Stein
Genus
Prorocentrum balticum (Lohmann) Loeblich
Prorocentrum compressum (Bailey) Abé ex Dodge
Prorocentrum micans Ehrenberg
Prorocentrum triestinum Schiller
Order Dinophysiales Lindemann 1928
Family Dinophysiaceae Stein 1883
Genus
Dinophysis acuminata Claparède & Lachmann
Dinophysis caudata Saville-Kenk
*Dinophysis cuneus (Schütt) Abé
*Dinophysis hastata Stein
*Dinophysis mitra Schütt
Dinophysis ovum Schütt
Dinophysis rotundata Claparède & Lachmann
*Dinophysis schuettii Murray & Whitting
Dinophysis sp.
*Ornithocercus heteroporus Kofoid
*Ornithocercus magnificus Stein
*Ornithocercus quadratus Schütt
*Ornithocercus steinii Schütt
*Ornithocercus thumii (A.Schmidt) Kof. & Skogs.
Order Gonyaulacales F.J.R. Taylor 1980
Family Ceratiaceae Lindemann 1928
Genus
Ceratium arietinum Cleve
*Ceratium azoricum Cleve
Ceratium candelabrum (Ehrenberg) Stein
Ceratium contortum (Gourret) Cleve
Ceratium deflexum (Kofoid) Jörgensen
Ceratium extensum (Gourret) Cleve
Ceratium furca (Ehrenberg) Claparède & Lachmann
Ceratium fusus (Ehrenberg) Dujardin
Ceratium gibberum Gourret
Ceratium horridum (Cleve) Gran
*Ceratium horridum var. claviger (Kofoid) Graham & Bronikowski
Ceratium inflatum (Kofoid) Jörgensen
Ceratium lineatum (Ehrenberg) Cleve
*Ceratium longirostrum Gourret
Ceratium lunula Schimper
Ceratium massiliense (Gourret) Jörgensen
Ceratium pentagonum Gourret
Ceratium platycorne Daday
*Ceratium praelongum (Lemmermann) Kofoid
Ceratium ranipes Cleve
Ceratium trichoceros (Ehrenberg) Kofoid
Ceratium tripos (O.F.Müller) Nitzsch
Ceratium vultur Cleve
Family Ceratocoryaceae Lindemann 1928
Genus
*Ceratocorys gourretii Paulsen
Ceratocorys horrida Stein
Family Gonyaulacaceae Lindemann
Genus
Gonyaulax polyedra Stein
Gonyaulax polygramma Stein
Gonyaulax spinifera (Claparède & Lachmann) Dissing
Family Pyrophaceae Lindemann 1928
Genus
Pyrophacus horologium Stein
*Pyrophacus steinii (Schiller) Wall & Dale
Family Oxytoxaceae
Genus
*Oxytoxum constrictum (Stein) Bütschli 1885
*Oxytoxum milneri Murray & Whitting 1899
*Oxytoxum reticulatum (Stein) Schütt
*Oxytoxum sceptrum (Stein) Schröder 1906
*Oxytoxum scolopax Stein 1883
*Oxytoxum tesselatum(Stein) Schütt 1895
Order Pyrocystales
Family Pyrocystaceae
Genus
*Pyrocystis hamulus Cleve
*Pyrocystis robusta Kofoid
Order Peridiniales
Family Peridiniaceae
Genus
*Dissodium asymmetricum (Morgin) Loeblich
Family Goniodomataceae
Genus
Goniodoma polyedricum (Pouchet) Jörgensen
Goniodoma sphaericum (Murray & Whitting)
Family Podolampaceae Lindemann 1928
Genus
*Podolampas bipes Stein
*Podolampas palmipes Stein
Podolampas spinifera Okamura
Family Protoperidiniaceae F.J.R. Taylor 1987
Genus
Protoperidinium brochii (Kofoid et Swezy) Balech
Protoperidinium cerasus (Paulsen) Balech
Protoperidinium claudicans (Paulsen) Balech
Protoperidinium conicoides (Paulsen) Balech
Protoperidinium conicum (Gran) Balech
Protoperidinium curvipes (Ostenfeld) Balech
Protoperidinium depressum (Bailey) Balech
Protoperidinium divergens (Ehrenberg) Balech
Protoperidinium marielebouriae (Paulsen) Balech
Protoperidinium nipponicumAbé
*Protoperidinium oblongum (Aurivillius) Parke & Dodge 1976
*Protoperidinium obtusum (Karsten) Parke & Dodge 1976
Protoperidinium oceanicum (vanHöffen) Balech
*Protoperidinium oviforme (Dangeard) Balech 1974
Protoperidinium pallidum (Ostenfeld) Balech
Protoperidinium pellucidum Bergh
Protoperidinium pentagonum (Gran) Balech
*Protoperidinium quinquecorne (Abé) Balach 1974
Protoperidinium roseum (Paulsen) Balech
*Protoperidinium steinii (Jörgensen) Balech 1974
*Protoperidinium thorianum (Paulsen) Balech 1973 Protoperidinium sp.
Order Gymnodiniales Lemmermann 1910
Family Gymnodiniaceae Lankester 1885
Genus
*Pseliodinium vaubanii Sournia 1972
Order Ptychodiscales
Family Ptychodiscaceae
Genus
*Ptychodiscus noctiluca Stein 1883
>
Description of Unrecorded Dinoflagellates
Genus Dinophysis Ehrenberg 1840
Dinophysis cuneus (Schutt) Abe 1967 (
Synonym:
References: Steidinger et al. 1967, pp 28–29, pl. Ⅰ. f; Dodge 1985, p 19; Fujioka 1990, pp 42–43, fig. 11; Steidinger and Tangen 1996, pp 438–439, pl. 14.
Specimen examined: Slide LJB2006-01 at the National Institute of Biological Resources (NIBR), Incheon.
Description: Epitheca looks like a dome and is relatively broad, but hypotheca is narrow like a reversed triangle. Cell surface has irregular reticulate ornamentation. This species is tropical or subtropical.
Size: Length 83–96 μm, width 96–116 μm.
Occurrence: Sep 2006 (J8 Gosan); Sep Nov Dec 2007 (J14 Seongsan).
Distribution: Oceanic, tropical species; worldwide distribution.
Dinophysis hastata Stein 1883 (
Synonym:
References: Dodge 1982, p 47, 49, fig. 4C; Dodge 1985, p 19; Taylor 1987, p 31, fig. 2.4a; Fujioka 1990, pp 44–45, fig. 5; Steidinger and Tangen 1996, pp 432–433, pl. 12.
Specimen examined: Slide LJB2006-02 at the NIBR, Incheon.
Description: Cell shape is subovate in later view with characteristic elongated triangular sail at the posterior end of hypotheca, ventral of the midline. Hypotheca round, deepest around the middle and the posterior end is narrower than anterior end.
Size: Length 83–84 μm, width 66–76 μm.
Occurrence: Dec 2006 (J7 Chagwido); Jan 2007 (J14 Seongsan); Aug 2007 (J13 Pyoseon); Oct 2007 (J9 Hamo).
Distribution: Mainly neritic species; distributed in temperate to tropical waters.
Dinophysis mitra Schutt 1895 (
Synonym:
References: Fujioka 1990, pp 44–45, fig. 8; Steidinger and Tangen 1996, pp 438–439, pl. 14.
Specimen examined: Slide LJB2008-06 at the NIBR, Incheon.
Description: Cell forms a reverse triangle in side view. The epitheca looks like a dome, and the hypotheca is slightly concave under the sulcus forming a slightly convex end in lateral view. This is a known toxic species.
Size: Length 60–70 μm.
Occurrence: Oct 2008 (J9 Hamo); Sep Nov 2008 (J14 Seongsan); Nov 2008 (J13 Pyoseon).
Distribution: Neritic and oceanic species; distributed in temperate to tropical waters.
Dinophysis schuettii Murray & Whitting 1899 (
Synonyms:
References: Wood 1963, pp 7–8, fig. 17; Fujioka 1990, pp 46–47, fig. 1; Steidinger and Tangen 1996, pp 432–433, pl. 12.
Specimen examined: Slide LJB2009-14 at the NIBR, Incheon.
Description: Spherical shape with a relatively small size in lateral outline. The sulcal list is supported by very sharp, rigid ribs. Posterior end of hypotheca has another sharp, rigid rib like spine.
Size: Cell size without list 40–45 μm.
Occurrence: Dec 2007 (J14 Seongsan); Oct 2008 (J9 Hamo).
Distribution: Oceanic, tropical species; worldwide distribution.
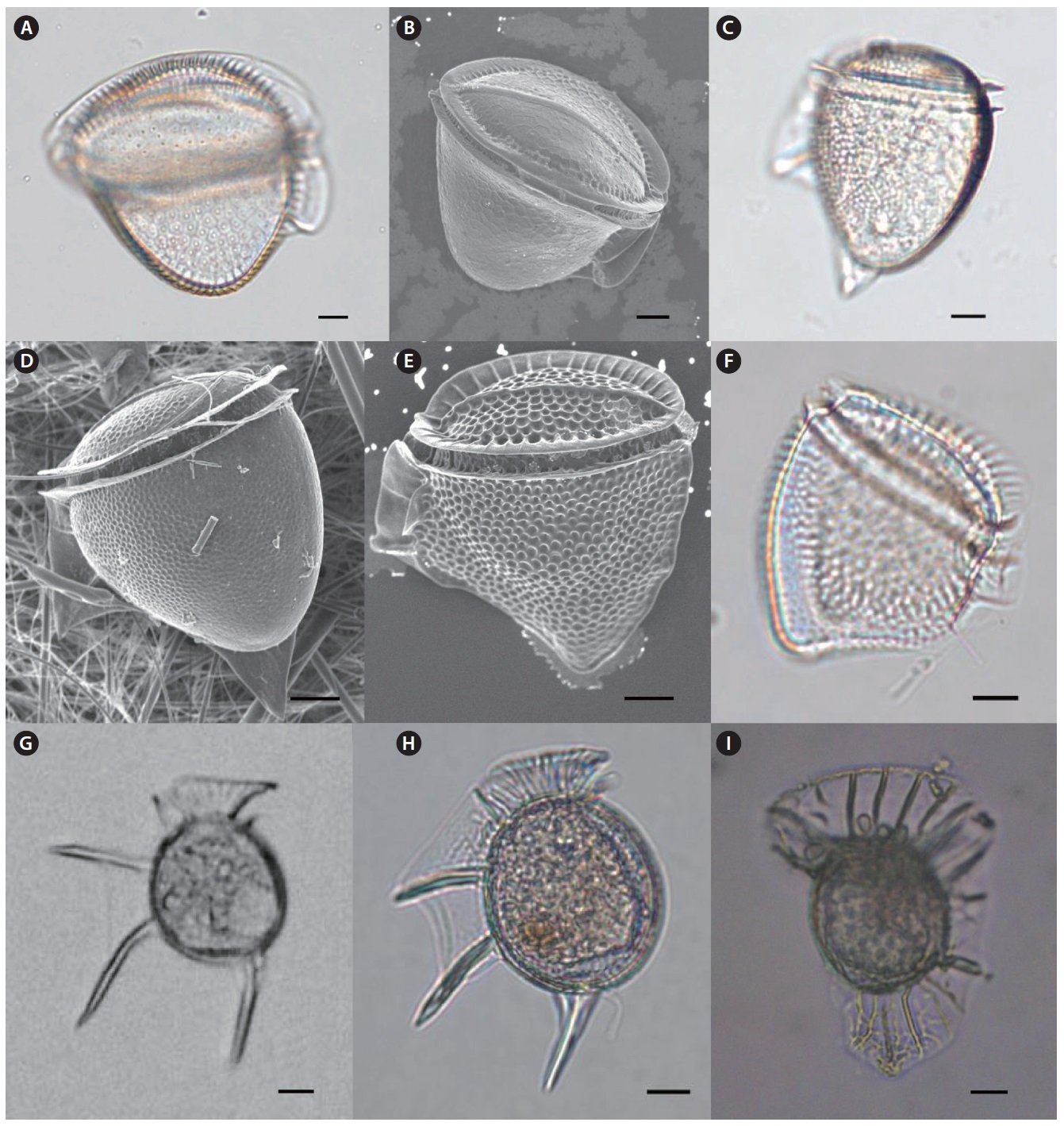
Genus Ornithocercus Stein 1883
Ornithocercus heteroporus Kofoid 1907 (
Synonyms:
References: Fujioka 1990, pp 46–47, fig. 7; Steidinger and Tangen 1996, pp 435–436, pl. 13; Jyothibabu et al. 2008, fig. 13c.
Specimen examined: Slide LJB2007-04 the NIBR, Incheon.
Description: Small compared to other species within the same genus. Cell is spherical, and the epitheca is much eliminated. Girdle list shaped like a skirt spreading anteriorly. The sulcal list is very widely spreading with a wide list below the sulcus.
symbiont, is frequently observed inside of the girdle list.
Size: Length 50–55 μm.
Occurrence: Nov 2006 (J7 Chagwido); Jan 2007 (J2 Samyang).
Distribution: Oceanic, tropical species; worldwide distribution.
Ornithocercus magnificus Stein 1883 (
Synonyms:
References: Hallegraeff and Jeffrey 1984, p 69, fig. 17; Dodge 1985, p 28; Fujioka 1990, pp 46–47, fig. 8; Steidinger and Tangen 1996, pp 435–436, pl. 13; Fernandez and García 1998, pp 557–558, fig. 38; Jyothibabu et al. 2008, fig. 13a.
Specimen examined: Slide LJB2006-03 at the NIBR, Incheon.
Description: Well known as a tropical planktonic species indicator. The girdle list is very large with a spreading anterior skirt-like wing. The sulcal list is supported by radial ribs. The girdle list characterizes a convex and concave outline compared to other
Size: Length 88–100 μm.
Occurrence: Feb Sep Oct Nov Dec 2006 (J7 Chagwido); Aug Sep Dec 2007 (J14 Seongsan); Aug Dec 2007 (J12 Namwon); Aug Oct 2007 (J9 Hamo); Aug 2007 (J6 Hallim).
Distribution: Oceanic, tropical species; worldwide distribution.
Ornithocercus quadratus Schutt 1900 (
Synonyms:
References: Dodge 1985, p 29; Steidinger and Tangen 1996, pp 435–436, pl. 13; Fernandez and García 1998, pp 557-558, fig. 36; Taylor et al. 2008, pp 410–411, fig. 2d; Jyothibabu et al. 2008, fig. 13b.
Specimen examined: Slide LJB2007-02 at the NIBR, Incheon.
Description: Characteristic wide girdle list and quadrated list of the sulcus. This species also has
Size: Length 122 μm, width 91 μm.
Occurrence: Nov 2006 (J7 Chagwido); Jan 2007 (J2 Samyang); Nov 2007 (J14 Seongsan).
Distribution: Oceanic, tropical species.
Ornithocercus steinii Schutt 1900 (
Synonym:
References: Steidinger et al. 1967, pp 30–31, pl. Ⅱ-c; Hallegraeff and Jeffrey 1984, p 67, fig. 6; Fujioka 1990, pp 48–49, fig. 5; Steidinger and Tangen 1996, p 435, 437, pl. 13; Fernandez and García 1998, pp 557–558, fig. 37.
Specimen examined: Slide LJB2006-05 at the NIBR, Incheon.
Description: Girdle list is supported by rigid ribs. Sulcal list has several radial ribs with round outline.
Size: Length 116–166 μm, width 111–123 μm.
Occurrence: Feb Aug Sep Oct Nov Dec 2006 (J7 Chagwido); Aug Sep 2007 (J14 Seongsan); Aug 2007 (J6 Hallim); Oct 2007 (J9 Hamo).
Distribution: Oceanic, tropical species; worldwide distribution.
Ornithocercus thumii (A. Schmidt) Kof. & Skogs. 1928 (
References: Fujioka 1990, pp 48–49, fig. 6; Steidinger and Tangen 1996, pp 435–437, pl. 13; Jyothibabu et al. 2008, fig. 13d.
Specimen examined: Slide LJB2007-03 at the NIBR, Incheon.
Description: Girdle list spreads anteriorly like a skirt and the sulcus is supported by ribs with regular intervals. The outline of sulcal list is distinctively divided into three parts.
Size: Length 120 μm, width 87 μm.
Occurrence: Mar 2007 (J9 Hamo); Aug 2007 (J7 Chagwido); Aug 2007 (J6 Hallim); Sep 2007 (J14 Seongsan).
Distribution: Neritic/oceanic species; tropical species; worldwide distribution.
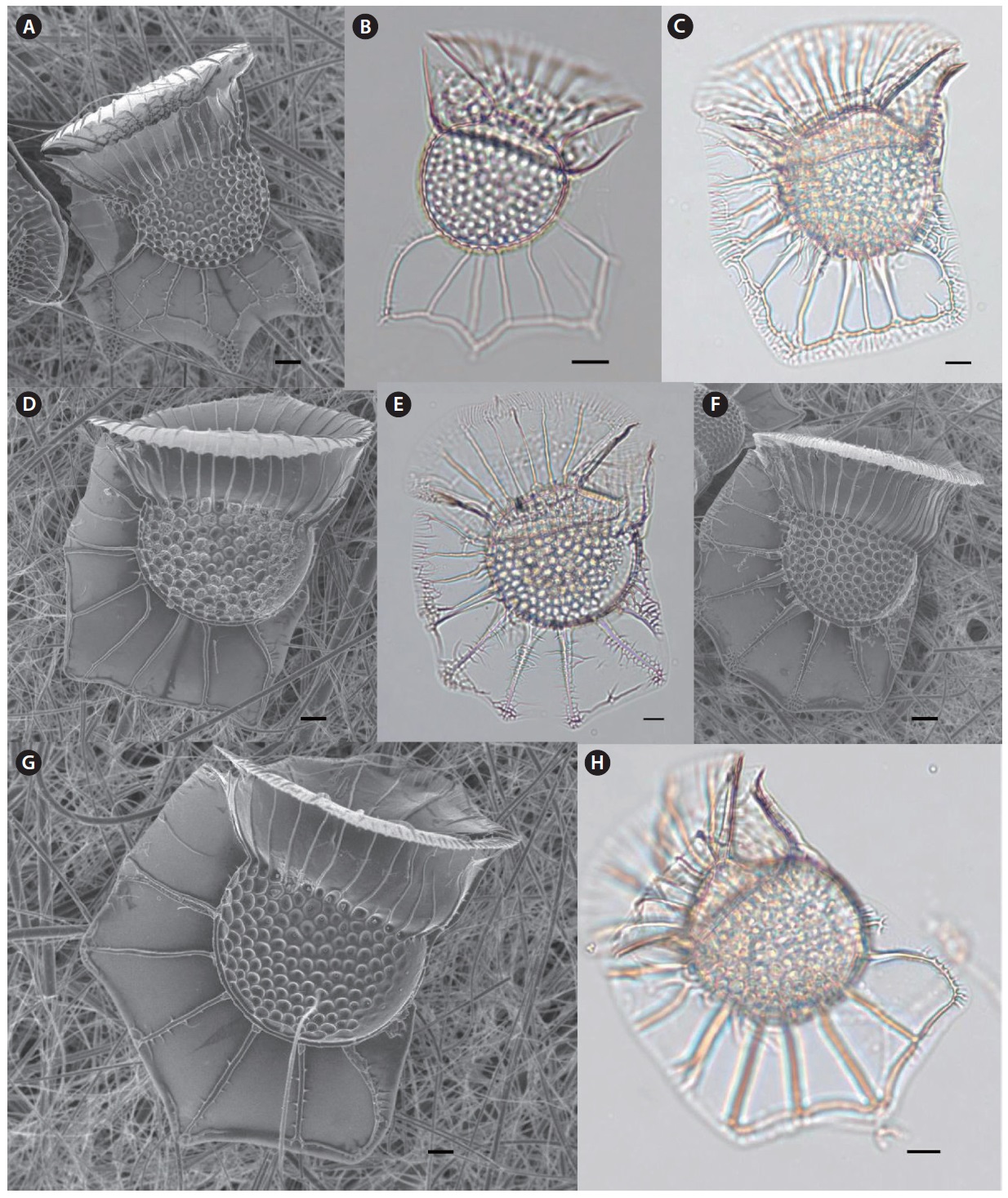
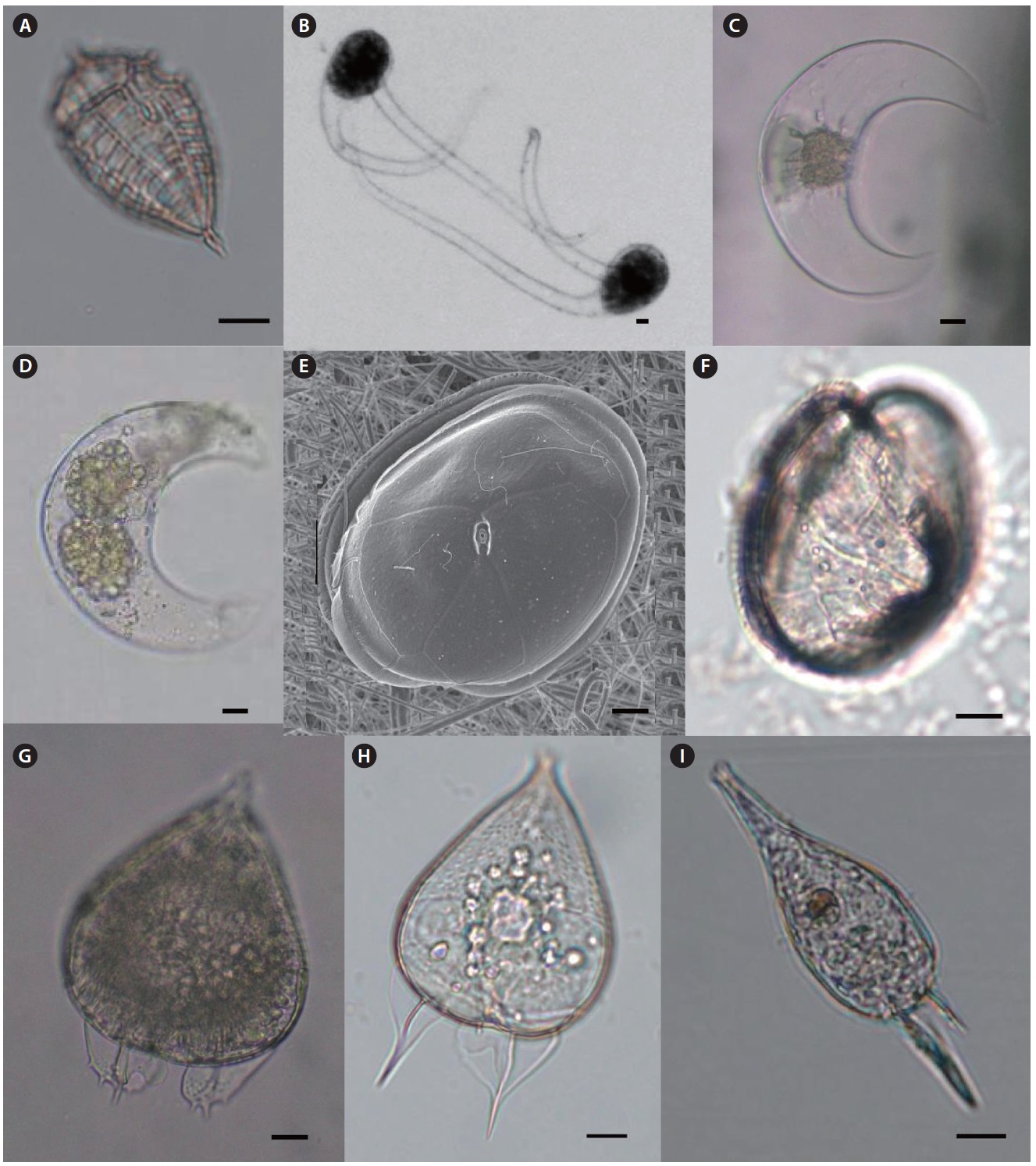
Genus Ceratium Schrank 1793
Ceratium azoricum Cleve 1900 (
References: Dodge 1982, pp 232–233, fig. 29, F; Dowidar 1983, p 523, 539, pl. Ⅳ, fig. 7; Fujioka 1990, pp 50–51, fig. 4.
Specimen examined: Slide LJB2007-05 at the NIBR, Incheon.
Description: Cell is relatively small and dorso-ventrally flattened. Anterior horn short and broad, hypotheca rounded with the broad posterior horns pointing in an anterior direction. Posterior horns with pointed tips.
Size: Length 100–110 μm, width 97 μm.
Occurrence: Aug Sep 2007 (J14 Seongsan).
Distribution: Found in Mexico Current; distributed in temperate and tropical areas.
Ceratium horridum var. claviger (Kofoid) Graham & Bronikowski (
References: Fujioka 1990, pp 54–55, fig. 6.
Specimen examined: Slide LJB2008-01 at the NIBR, Incheon.
Description: This species is medium sized and fairly delicate with short open-ended horns. The epitheca is higher than the hypotheca in body length. Anterior horn width is equal and straight to a pointed end. The left and right horns of the hypotheca are nearly parallel but bent at the middle of both horns. Both ends of the hypotheca horn are flattened.
Size: Body length 50–60 μm without horn.
Occurrence: Aug 2008 (J9 Hamo).
Distribution: Found in Kuroshio Current.
Ceratium longirostrum Gourret 1883 (
Synonyms:
References: Dodge 1982, p 231, 233, fig. 29D; Carbonell 1982, pp 80–81, fig. 6; Dowidar 1983, p 521, 537, pl. Ⅱ, fig. 8; Fernandez and García 1998, p 542, fig. 4.
Specimen examined: Slide LJB2008-02 at the NIBR, Incheon.
Description: This species is very similar to
Size: Length 494–514 μm, width 34–37 μm.
Occurrence: Apr 2006 (J7 Chagwido); Aug Sep 2006, Aug 2007, Sep 2008 (J14 Seongsan).
Distribution: Found in Atlantic Ocean.
Ceratium praelongum (Lemmermann) Kofoid 1907 (
Synonym:
References: Wood 1963, pp 40–41, fig. 148; Carbonell 1982, p 77, 81, fig. 2; Dowidar 1983, p 517, 536, pl. Ⅰ, fig. 1; Taylor 1987, pp 34–35, fig. 2.6d; Fujioka 1990, pp 58–59, fig. 3; Steidinger and Tangen 1996, p 473, 477, pl. 25.
Specimen examined: Slide LJB2007-07 at the NIBR, Incheon.
Description: The round end of the epitheca like a duck foot with no anterior horn. However the left and right horns of the hypotheca are thick and strait to posterior ends. The right horn is longer than the left.
Size: Length 250–260 μm.
Occurrence: Feb 2007 (J7 Chagwido).
Distribution: Oceanic, tropical species; worldwide distribution.
Genus Ceratocorys Stein 1883
Ceratocorys gourretii Paulsen 1931 (
Synonyms:
References: Fujioka 1990, pp 62–63, fig. 3.
Specimen examined: Slide LJB2007-10 at the NIBR, Incheon.
Description: Epitheca is short and slightly convex in lateral view, but hypotheca is round and ovate. The sulcal list is very short and rigid. The hypotheca has no list but five spines with a rigid list.
Size: Length 80–90 μm, width 75–85 μm.
Occurrence: Aug Oct Nov 2007 (J14 Seongsan); Sep 2007 (J2 Samyang); Nov 2007 (J8 Gosan); Aug Oct Nov 2007 (J14 Seongsan); Sep 2007 (J2 Samyang); Nov 2007 (J8 Gosan); Nov 2007 (J12 Namwon).
Distribution: Tropical or subtropical species.
Genus Pyrophacus Stein 1883
Pyrophacus steinii (Schiller) Wall & Dale 1971 (
Synonyms:
References: Fujioka 1990, pp 68–69, fig. 7; Fukuyo et al. 1990, pp 116–117; Steidinger and Tangen 1996, pp 522–523, pl. 46.
Specimen examined: Slide LJB2008-13 at the NIBR, Incheon.
Description: Cell is dorso-ventrally strongly flattened like a lens shape. The plate of hypotheca is Po, 6-8”, 0-2a, 9-14”, 11- 12”’, 3p, 2-3””.
Size: Cell width 110–120 μm.
Occurrence: Aug 2008 (J1 Bukchon); Jul Nov 2008(J6 Hallim); Oct 2008 (J14 Seongsan); Nov 2008 (J7 Chagwido).
Distribution: Widely distributed from temperate to tropical areas worldwide.
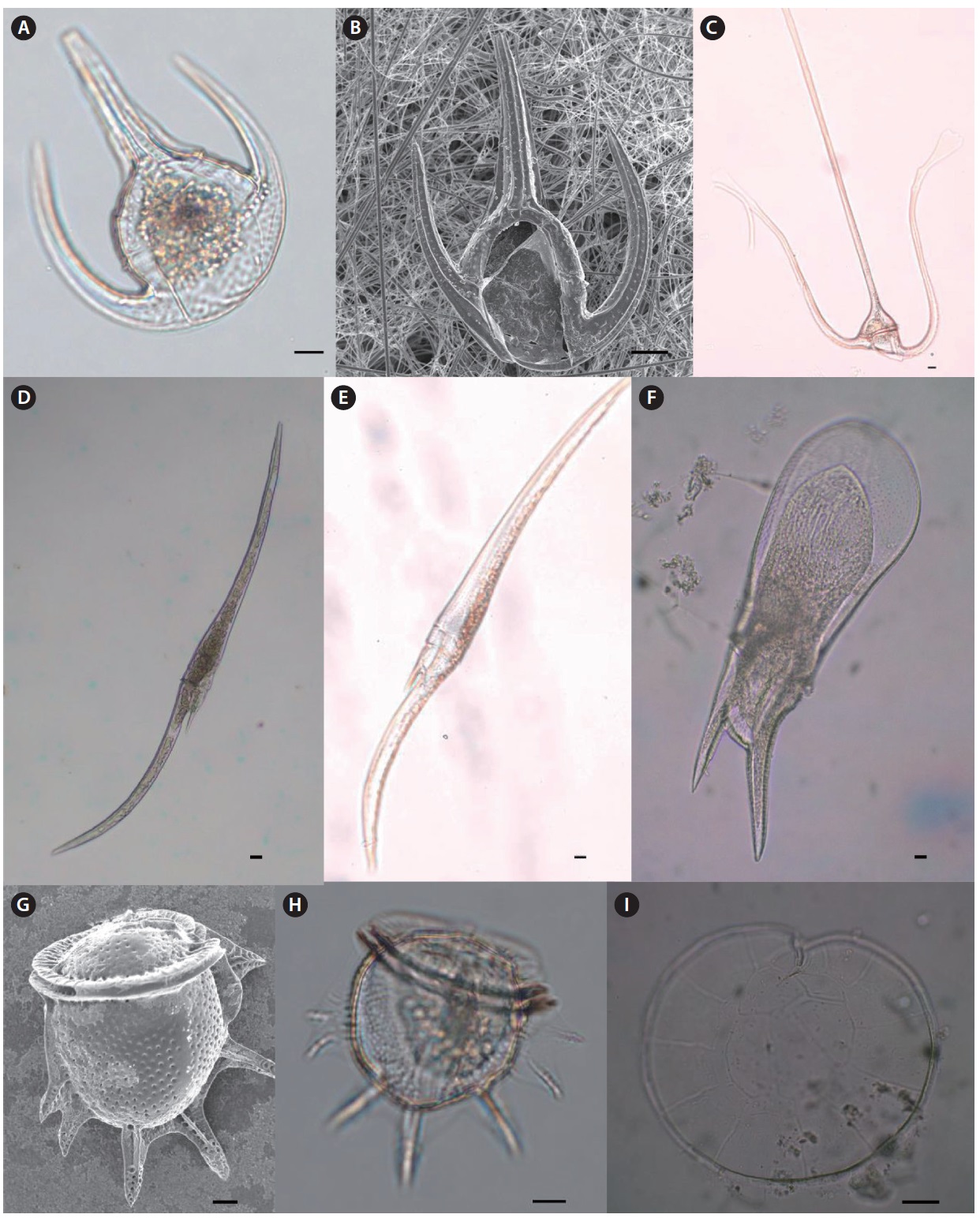
Genus Oxytoxum Stein 1883
Oxytoxum constrictum (Stein) Butschli 1885 (
References: Wood 1963, p 44, 47, fig. 163; Dodge 1985, p 107; Gomez et al. 2008, figs. 15–16.
Specimen examined: Slide LJB2006-08 at the NIBR,
Incheon.
Description: The cell is spindle in form. In lateral view, epitheca is pyramid shaped and one-third of total body length. The middle part of hypotheca has a hole-like depression. The end of the hypotheca is pointed.
Size: Length 40–60 μm.
Occurrence: Oct 2006 (J14 Seongsan).
Distribution: Tropical species; reported in Mediterranean Sea and around coral reefs.
Oxytoxum milneri Murray & Whitting 1899 (
Synonym:
References: Wood 1963, pp 46–47, fig. 173; Dodge 1982, p 243, 246, fig. 32G; Dodge 1985, p 109; Gomez et al. 2008, fig. 9.
Specimen examined: Slide LJB2007-14 at the NIBR, Incheon.
Description: Cell is a spindle form. Both ends of epitheca and hypotheca are very sharp like a stinger.
Size: Length 70–75μm, width 37μm.
Occurrence: Aug Oct 2007, Aug 2009 (J14 Seongsan); Oct 2007 (J4 Iho).
Distribution: Reported in Atlantic Ocean, Mediterranean Sea, Indian Ocean, Tasmanian Sea, Caribbean Sea, Florida Strait, and Brazil Sea.
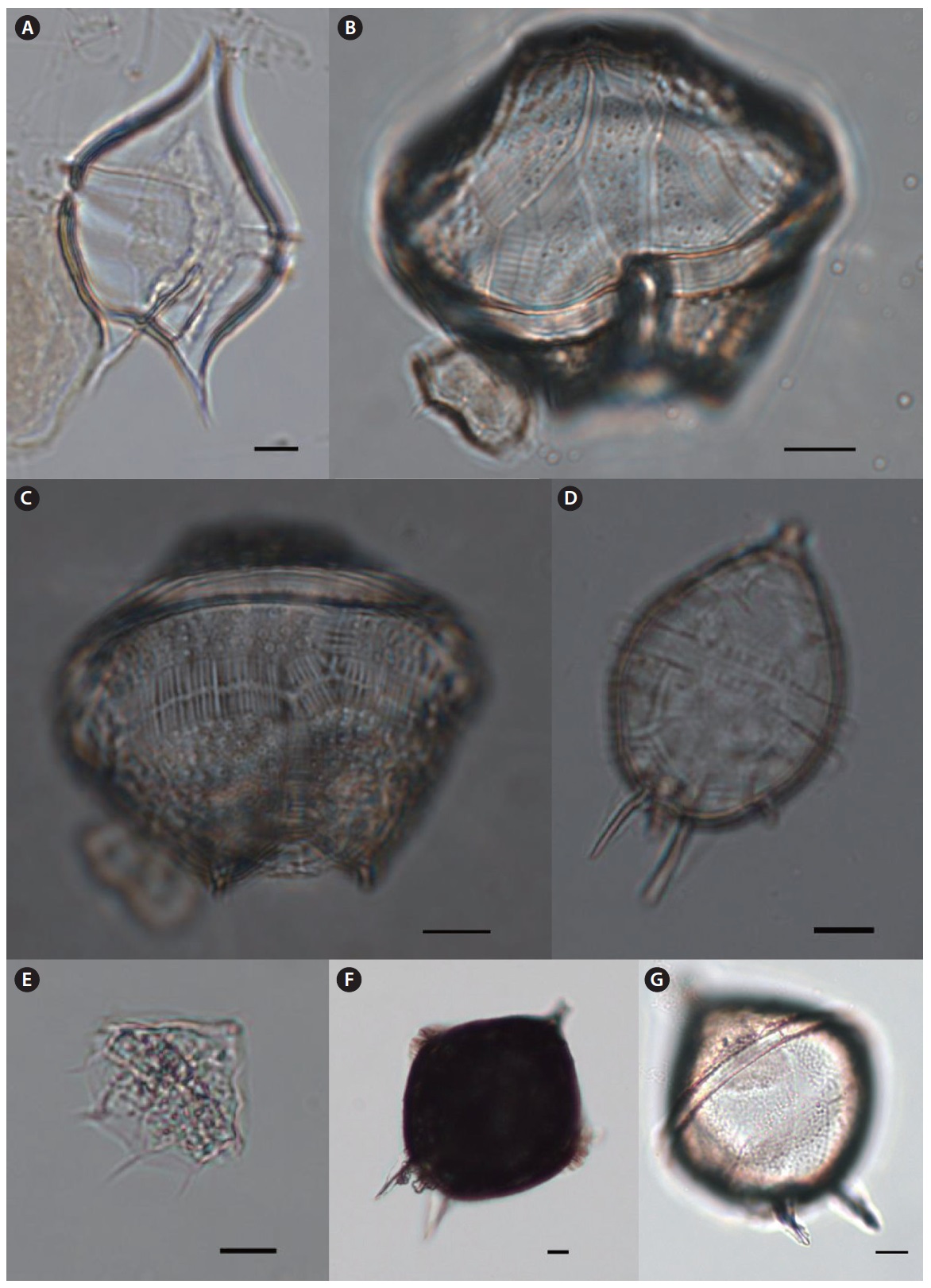
Oxytoxum reticulatum (Stein) Schutt (
Synonyms:
References: Dodge 1982, p 243, 245, fig. 32F.
Specimen examined: Slide LJB2009-22 at the NIBR, Incheon.
Description: Cell elongated with hypotheca at least twice as long as epitheca. Epitheca short and conical with longitudinal lines radiating from the apex. Thecal plates have many poroids. Hypotheca width is broadly wide and rounded anteriorly tapering to a point at the antapex.
Size: Length 53 μm.
Occurrence: Aug 2009 (J14 Seongsan).
Distribution: Found in Mediterranean Sea and Atlantic Ocean.
Oxytoxum sceptrum (Stein) Schroder 1906 (
Synonyms:
References: Wood 1963, p 47, 49, fig. 179; Dodge 1985, p 111.
Specimen examined: Slide LJB2009-23 at the NIBR, Incheon.
Description: Cell elongated with pointed apices. Epitheca short and conical with sharp spine at the apex. Hypotheca is slightly long and broad with pointing spine at the antapex.
Size: Length 62 μm, width 22 μm.
Occurrence: Aug 2009 (J14 Seongsan).
Distribution: Reported in Indian Ocean.
Oxytoxum scolopax Stein 1883 (
References: Steidinger et al. 1967, pp 38–39, pl. Ⅵ. f; Dodge 1982, p 243, 247, fig. 32H; Dodge 1985, p 108; Fujioka 1990, pp 64–65, fig. 10; Steidinger and Tangen 1996, p 519; Gomez et al. 2008, figs. 6–7; Taylor et al. 2008, pp 410-411, fig. 2j.
Specimen examined: Slide LJB2006-09 at the NIBR, Incheon.
Description: Cell spindle-shaped with acutely pointed ends. Hypotheca about three times the length of the epitheca. Hypotheca has small swelling at the base of the antapical point. Epitheca is tapered to apex.
Size: Length 56μm, width 15μm.
Occurrence: Oct 2006 (J14 Seongsan).
Distribution: Oceanic, tropical species; reported in Pacific and Atlantic Oceans.
Oxytoxum tesselatum (Stein) Schutt 1895 (
Synonyms:
References: Dodge 1985, p 113; Fujioka 1990, p 66–67, Fig. 2; Steidinger and Tangen 1996, p 517–518, pl. 45; Gomez et al. 2008, fig. 11.
Specimen examined: Slide LJB2007-13 at the NIBR, Incheon.
Description: Cell spindle-shaped with pointed apices. Epitheca conical with reticulated plate. Hypotheca tapering to the antapex with a sharp spine. The hypothecal plate has regularly rectangular ornamentations.
Size: Length 45–55μm, width 28μm.
Occurrence: Aug Nov 2007 (J14 Seongsan); Sep Oct 2007 (J7 Chagwido).
Distribution: Tropical species; reported in Atlantic Ocean.
Genus Pyrocystis Murray ex Haeckel 1890
Pyrocystis hamulus Cleve 1900 (
References: Dodge 1982, p 137, fig. 16I.
Specimen examined: Slide LJB2009-34 at the NIBR, Incheon.
Description: This genus has two forms of coccoid and thecate. The coccoid form is normally found as a cyst of the thecate form. The cysts are elongated and curved, with a central swelling containing the cytoplasm. Usually occur in pairs with attached form of the distal ends of the arms. The genus is known to occur mainly in warm water.
Size: Length 400–450 μm.
Occurrence: Feb 2007 (J7 Chagwido); Jan 2007 (J14 Seongsan).
Distribution: Tropical species; Reported in English Channel and in southern bay of Scotland.
Pyrocystis robusta Kofoid 1907 (
References: Fujioka 1990, pp 40–41, fig. 4.
Specimen examined: Slide LJB2006-14 at the NIBR, Incheon.
Description: This species is lunate in form with jelly-like cyst body. Cytoplasm is located in the center of the cyst. Similar to
Size: Length 90–100 μm.
Occurrence: Feb 2007 (J9 Hamo).
Distribution: Reported in Kuroshio Current.
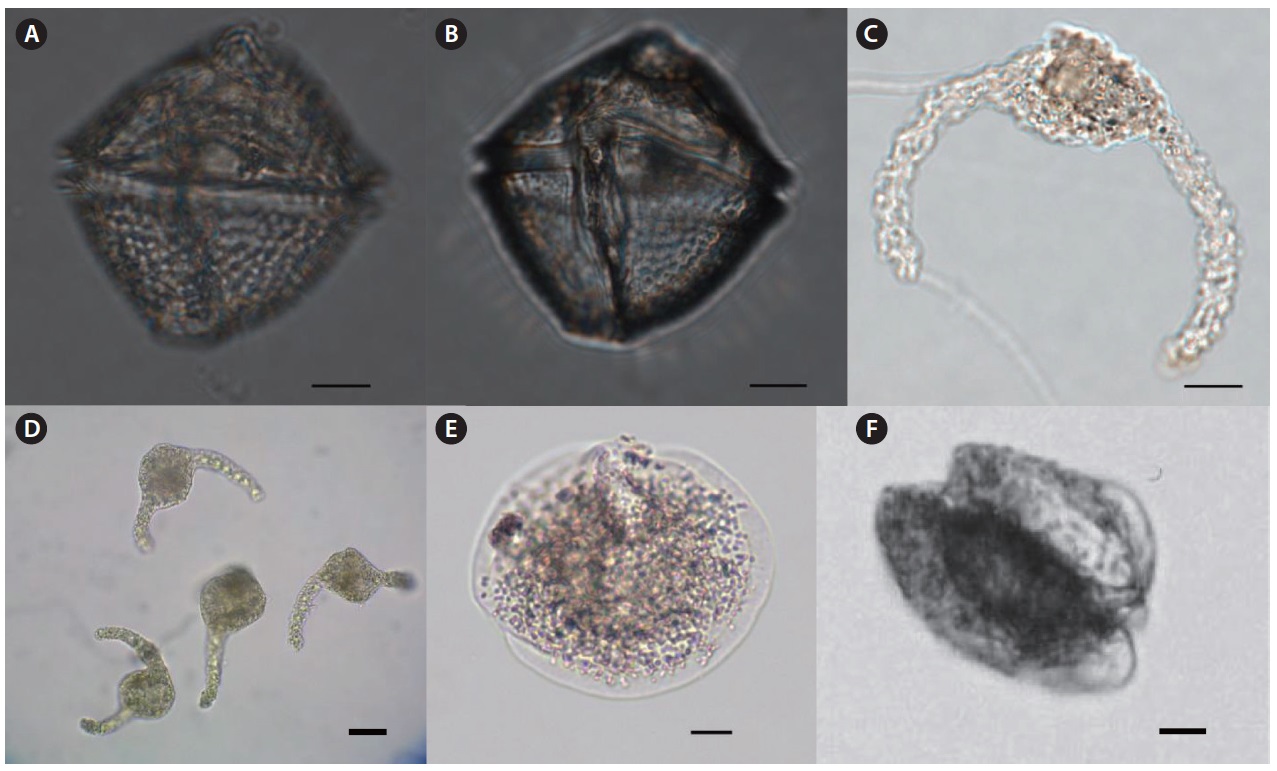
Genus Dissodium Abe 1941
Dissodium asymmetricum (Morgin) Loeblich 1970 (
Synonyms:
References: Subrahmanyan 1971, p 156, 158, pl. Ⅰ, figs. 10–11, 15, pl. Ⅱ, figs. 1–4, 6, 8, 10, 12–15; Dodge 1982, p 153, 157, fig. 18F-G; Skaloud et al. 2006, p 726, fig. 38.
Specimen examined: Slide LJB2007-06 at the NIBR, Incheon.
Description: Circular in apical view and lenticular to globular in ventral view. Epitheca with convex sides ending in a small apical projection surrounding the apical pore. Intercalary striae may be present. The girdle is equatorial and not displaced or excavated but bordered by a list, typically without supporting spines. Hypotheca equal in depth to epitheca with convex sides.
Size: Length 30–50 μm, width 48–70 μm.
Occurrence: Aug 2007 (J14 Seongsan).
Distribution: Reported in Baltic Sea, Atlantic Ocean, Mediterranean Sea, Indian Ocean, and Mexico Bay.
Genus Podolampas Stein 1883
Podolampas bipes Stein 1883 (
References: Steidinger et al. 1967, pp 34–35, pl. Ⅳ, a; Dodge 1985, p 117; Taylor 1987, pp 58–59, fig. 2.16d; Fujioka 1990, pp 66–67, figs. 4–5; Steidinger and Tangen 1996, p 399, 534, pl. 7.
Specimen examined: Slide LJB2006-10 at the NIBR, Incheon.
Description: The genus is characterized by the presence of a distinct apical horn ending in an apical poreand is generally pyriform in shape bearing two antapical spines. Cell looks like a slightly depressed chestnut. Epitheca is triangular, ending in two unequal antapical spines. Hypotheca is half spherical form, with two left and right wing-like structures with sharp and strong spines at the antapical end.
Size: Length 95–106 μm, width 55–77 μm.
Occurrence: Feb 2006 (J4Iho); Aug 2006 (J14 Seongsan); Apr Oct Nov 2006 (J7 Chagwido); Feb 2007 (J5 Aewol).
Distribution: Oceanic and worldwide tropical species.
Podolampas palmipes Stein 1883 (
References: Dodge 1982, p 251, 254, fig. 33I; Fujioka 1990, pp 66–67, fig. 7; Steidinger and Tangen 1996, pp 534–535, pl. 50.
Specimen examined: Slide LJB2006-11 at the NIBR, Incheon.
Description: Cell pyriform, narrow, ending in two unequal antapical spines. The epitheca is drawn out into a long, slender neck. The right-hand spine is much shorter than the left antapical spine.
Size: Length 88–97 μm, width 26 μm.
Occurrence: Sep 2006 (J2 Samyang); Oct Nov 2006 (J7 Chagwido); Aug Oct Nov 2006 (J14 Seongsan); Nov 2006 (J1 Bukchon); Nov 2006 (J8 Gosan); Nov 2006 (J4 Iho); Nov 2006 (J13 Pyoseon); Oct 2006 (J10 Hwasun); Aug 2007 (J14 Seongsan); Sep 2007 (J2 Samyang).
Distribution: Oceanic, worldwide tropical species.
Genus Protoperidinium Bergh 1881
Protoperidinium oblongum (Aurivillius 1898) Parke & Dodge 1976 (
Synonyms:
References: Steidinger et al. 1967, pp 42–43, pl. Ⅷ, b; Dodge 1982, pp 179–180, fig 20B–D; Dodge 1985, p 58; Steidinger and Tangen 1996, p 539, p 541, pl. 52; Evagelopoulos 2002, p 44, 49, 51, figs. 7–9, 36; Skaloud et al. 2006, p 726, fig. 47; Okolodkov 2008, pp 142–143, pl. 15, fig. 6.
Specimen examined: Slide LJB2009-25 at the NIBR, Incheon.
Description: Cell shape is similar to
Size: Length 92 μm, width 60 μm.
Occurrence: Sep 2009 (J7 Chagwido).
Distribution: Worldwide distributed from neritic to oceanic areas.
Protoperidinium obtusum (Karsten) Parke & Dodge 1976 (
Synonyms:
References: Dodge 1982, p 185, fig. 21C; Dodge 1985, p 59; Steidinger and Tangen 1996, pp 542–543, pl. 53; Evagelopoulos 2002, p 45, pp 51–52, figs. 17–19, 42–43; Okolodkov 2008, p 119, 121, pl. 6. figs. 8–10.
Specimen examined: Slide LJB2009-26 at the NIBR, Incheon.
Description: Cell length is similar to width, and it has a blunt apex, a rounded dorsal surface and is dorso-ventrally flattened. The hypotheca has two hollow antapical horns.
Size: Length 65–68 μm, width 65 μm.
Occurrence: Aug 2009 (J14 Seongsan).
Distribution: Tropical species from neritic to oceanic area; worldwide distribution.
Protoperidinium oviforme (P. Dangeard 1927) Balech 1974 (
References: Evagelopoulos 2002, pp 47–48, 52, fig. 49; Okolodkov 2008, pp 139–140, pl. 14, figs. 5–8.
Specimen examined: Slide LJB2009-27 at the NIBR, Incheon.
Description: Cell shaped generally similar to
Size: Length 76 μm, width 42 μm.
Occurrence: Aug 2009 (J14 Seongsan).
Distribution: Worldwide distribution.
Protoperidinium quinquecorne (Abe) Balach 1974 (
References: Dodge 1985, p 37; Fukuyo et al. 1990, pp 138–139.
Specimen examined: Slide LJB2009-28 at the NIBR, Incheon.
Description: A small and delicate species in the genus
Size: Length 40 μm, width 35 μm.
Occurrence: Aug 2009 (J14 Seongsan).
Distribution: Reported in red-tide areas and in shallow waters of tropical areas.
Protoperidinium steinii (Jorgensen 1899) Balech 1974 (
Synonyms:
References: Subrahmanyan 1971, p 200, pl. ⅩⅬ, figs. 1–13; Dodge 1982, p 197, 199, fig. 23C; Dodge 1985, p 65; Fujioka 1990, pp 68–69, fig. 3.
Specimen examined: Slide LJB2006-15 at the NIBR, Incheon.
Description: Cell pyriform with elongated apical horn and rounded hypotheca bearing two long three-winged spines. Epitheca and hypotheca are almost equal in length.
Size: Length 110–161 μm, width 80–119 μm.
Occurrence: Jan 2007 (J7 Chagwido).
Distribution: Worldwide distribution; reported around English Strait, Baltic Sea, Atlantic Ocean, Caribbean Sea.
Protoperidinium thorianum (Paulsen 1905) Balech 1973 (
Synonyms:
References: Dodge 1982, pp 175–176, fig. 19E; Dodge 1985, p 67; Steidinger and Tangen 1996, p 543, 546, pl. 53; Okolodkov 2008, pp 107–108, pl. 2, figs. 1–4; Morquecho et al. 2009, p 19, figs. 28–33.
Specimen examined: Slide LJB2009-29 at the NIBR, Incheon.
Description: Cell rhombic shaped; epitheca and hypotheca almost equal in length, with strong papillate or wavy thecal surface. Sides are rounded on ventral view. Sulcus straight, narrow, and not usually reaching the center of hypotheca. Girdle distinctively excavated.
Size: Length 60 μm, width 60 μm.
Occurrence: Aug 2009 (J14 Seongsan).
Distribution: Reported around English Strait, near Island Strait, Atlantic Ocean.
Genus Pseliodinium Sournia 1972
Pseliodinium vaubanii Sournia 1972 (
Synonym:
References: Steidinger et al. 1967, pp 32–33, pl. Ⅲ-c; Fujioka 1990, pp 42–43, fig. 1; Fukuyo et al. 1990, pp 60–61; Gomez 2007, pp 274–275, figs. 16–19.
Specimen examined: Slide LJB2006-12 at the NIBR, Incheon.
Description: Cell round shaped with two slightly curved arms. Girdle located in the center of cell and apparently distinguished. This species was deformed by physical force during the sampling process, which resulted in shrunken and curved form from
Size: Length 70–75 μm.
Occurrence: Aug 2006 (J8 Gosan); Feb Apr Nov 2006 (J7 Chagwido); Jan 2007 (J9 Hamo).
Distribution: Known to be a red-tide causative organism in warm waters; reported in the Kuroshio Current.
Genus Ptychodiscus Stein 1883
Ptychodiscus noctiluca Stein 1883 (
Synonyms:
References: Dodge 1982, p 113, fig. 13B-C; Taylor 1987, p 43, fig. 2.10c; Steidinger and Tangen 1996, p 462, 463, pl. 22; Gomez 2007, p 279, figs. 37–39.
Specimen examined: Slide LJB2006-13 at the NIBR, Incheon.
Description: Cell compressed apically, antapically like a shape of two sliced stones overlapped. Epicone flattened with a prominent heel on the ventral side, hypocone domed. Girdle horizontal, wide deep and bounded by substantial ridges or flaps. Sulcus starting on the heel and forming a groove in the epicone.
Size: Length 30–35 μm, width 70–82 μm.
Occurrence: Oct 2006 (J2 Samyang); Feb Nov 2006 (J7 Chagwido).
Distribution: Widely distributed from neritic to oceanic waters and from tropical to cold water areas.
About 153 dinoflagellates have been recorded in Korea waters. Shim (1994) described the highest number of species (n = 120). Shim et al. (1981) included descriptions of 49 species around Yeosu Bay. Han and Yoo (1983a, 1983b) described 61 species from Jinhae Bay. Yoo and Lee (1986) reported five species in the genus
Thirty-four species including one variety were newly recorded in Korean waters from the present study. An unrecorded species is one in which no taxonomic description is available in monographs or taxonomic studies. The morphological observations of the dinoflagellates collected in this study generally agreed with previous taxonomic descriptions from other geographical locations. However, the 34 newly recorded species included some species reported in a check list of ecological studies without taxonomic descriptions, including
Kim et al. (2008) reported seasonal variation in the genus
We have confirmed changes in the phytoplankton community in Korean waters by identifying tropical/newly recorded dinoflagellates around Jeju Island. These recent changes in species composition are greater than those reported previously. These changes may be the result of increasing sea surface temperature by global warming and expansion of the Kuroshio Current (Pang et al. 1996, Yeh and Kim 2010). Thus, tropical dinoflagellates could be planktonic evidence for global warming and marine ecosystem changes around Korea, but quantitative data based on the planktonic ecosystem are required to provide concrete evidence of climate change. An interdisciplinary study with long term monitoring is needed to explain such an ecosystem shift.