Rhodophyta is one of major groups in the number of species and diversity of the marine algal flora. The occurrence of Korean 5 red algal species is reported for the first time on the list of Korean marine algal flora based on morphology: Ceramium pacificum, Cumathanmnion serrulatum, Gayliella fimbriata, Leptofauchea rhodymenioides, Sorella pulchra. Ceramium pacificum from Korea is recognized by complete cortication, many adventitious branches in a radial arrangement, 7-8 periaxial cells, and plant length of 1-2 cm. Cumathanmnion serrulatum is characterized by cartilaginous single main axis with a prominent midrib, serrulate blade, many higher orders of bladelets on each blade, and tetrasporagia produced near the midrib acropetally and then outwardly. Gayliella fimbriata is featured by clavate gland cell and 5-7 periaxial cells. Leptofauchea rhodymenioides is characterized by erect with flattened, dichotomously branched fronds, 1-2 cortical cells loosely arranged, 2-3 cell medullar layers with large colourless cells. Sorella pulchrais recognized by short branches produced alternately pinnate manner from margins of axial, mostly polystromatic frond, and tetrasporangial sori on the center of branches.
Kang (1966) reported 403 species in the catalogue of Korean marine algal flora. Afterwards, many species have been added to the Korean algal inventory though monographic and floristic researches (Lee and Kang 1986, Lee 2008). Up to now, about 900 species have been recorded in the Korean marine algal flora (Boo and Ko 2012).
Rhodophyta is one of major groups in the number of species and diversity of the marine algal flora. It is attached to surfaces of rocks, ropes, or organisms, has various habitats from intertidal zone to subtidal region, and distributes in the world. Rhodophyta is a large assemblage of between 2,500 and 6,000 species in about 670 largely marine genera (Woelkerling 1990). In the list of Korean flora (Lee and Kang 2002), over 260 red algal species have been added after 247 red algal species reported by Kang (1966).
We collected 5 red algal species which are not listed on the Korean marine algal inventory. In this study, we report them as the first records from Korea based on morphological observations:
Plants were collected from the coasts of Korea. Taxonomic data were obtained from 5% formalin/seawater solution-preserved specimens. Materials were dissected by hand section using pith stick and razor blade. Materials were observed microscopically and stained with 1% aqueous aniline blue acidified with 0.1% diluted HCl. For permanent slides, the glycerin was exchanged with 20% corn syrup. Photomicrographs were taken by CCD camera (DMCe 5000, INS industry co. Ltd., Korea) and digital camera (D300s, Nikon, Tokyo, Japan; DP71, Olympus, Tokyo, Japan) attached to microscope (BX50, Olympus; BX51TRF, Olympus). Representative specimens examined in this study are deposited in the National Institute of Biological Resources, Ministry of Environment, Korea.
>
Ceramium pacificum (Collins) Kylin 1925. P. 61 (
Korean name: 잔가지비단풀(신칭)
Taxonomic position:
Phylum Rhodophyta
Class Florideophyceae
Order Ceramiales
Family Ceramiaceae
Genus
Lectotype: Phykotheka Universalis 302 (FH).
Type locality: Monterey, California.
Specimens examined: KOSPAL 0000126301, PKNU 0000126302 (Jumunjin: 26 Oct 2012).
Habitat: Epiphytic on other algae.
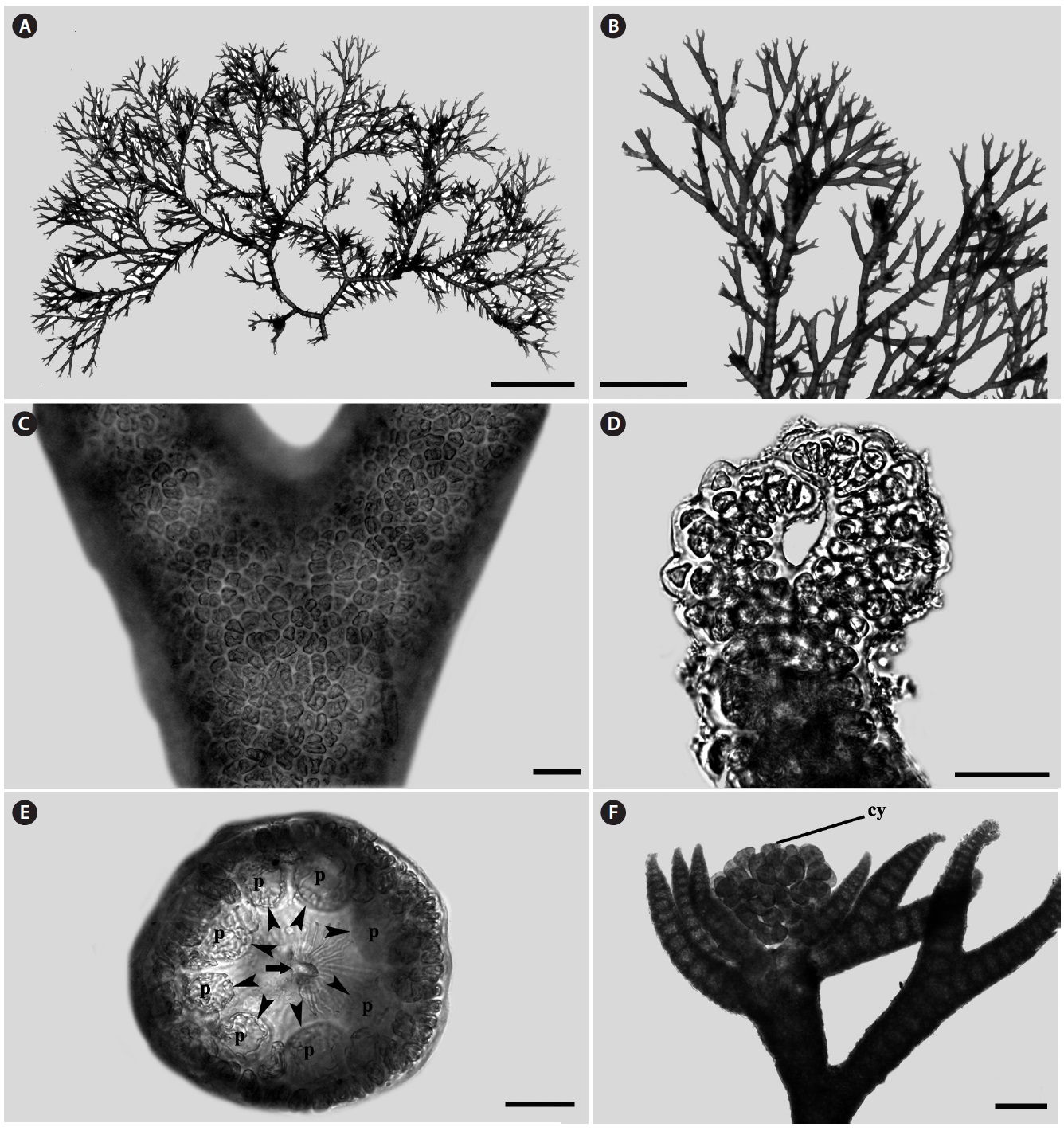
Morphology: Thalli up to 1-2 cm high (Fig. 1A), epiphytic on other algae, dichotomously branched (Fig. 1B), pink to light red in color and have many adventitious branches in a radial arrangement. Branching interval 7-14 nodes. Apex inrolled (Fig. 1D). Cortication complete (Fig. 1C) with 7-8 periaxial cells (Fig. 1E). Cystocarps produced near the apical portion, and surrounded by 5-7 involucral branchlets (Fig. 1F).
Remarks:
>
Cumathanmnion serrulatum (Harvey) M. J. Wynne & G. W. Saunders (
Korean name: 복바닷잎(신칭)
Taxonomic position:
Phylum Rhodophyta
Class Florideophyceae
Order Ceramiales
Family Delesseriaceae
Genus
Lectotype: FH (Yoshida 1998: 971).
Type locality: Hakodate, Japan.
Specimens examined: CUK9259 (Sokcho: 1 Feb 2012).
Habitat: Epilithic on bed rock.
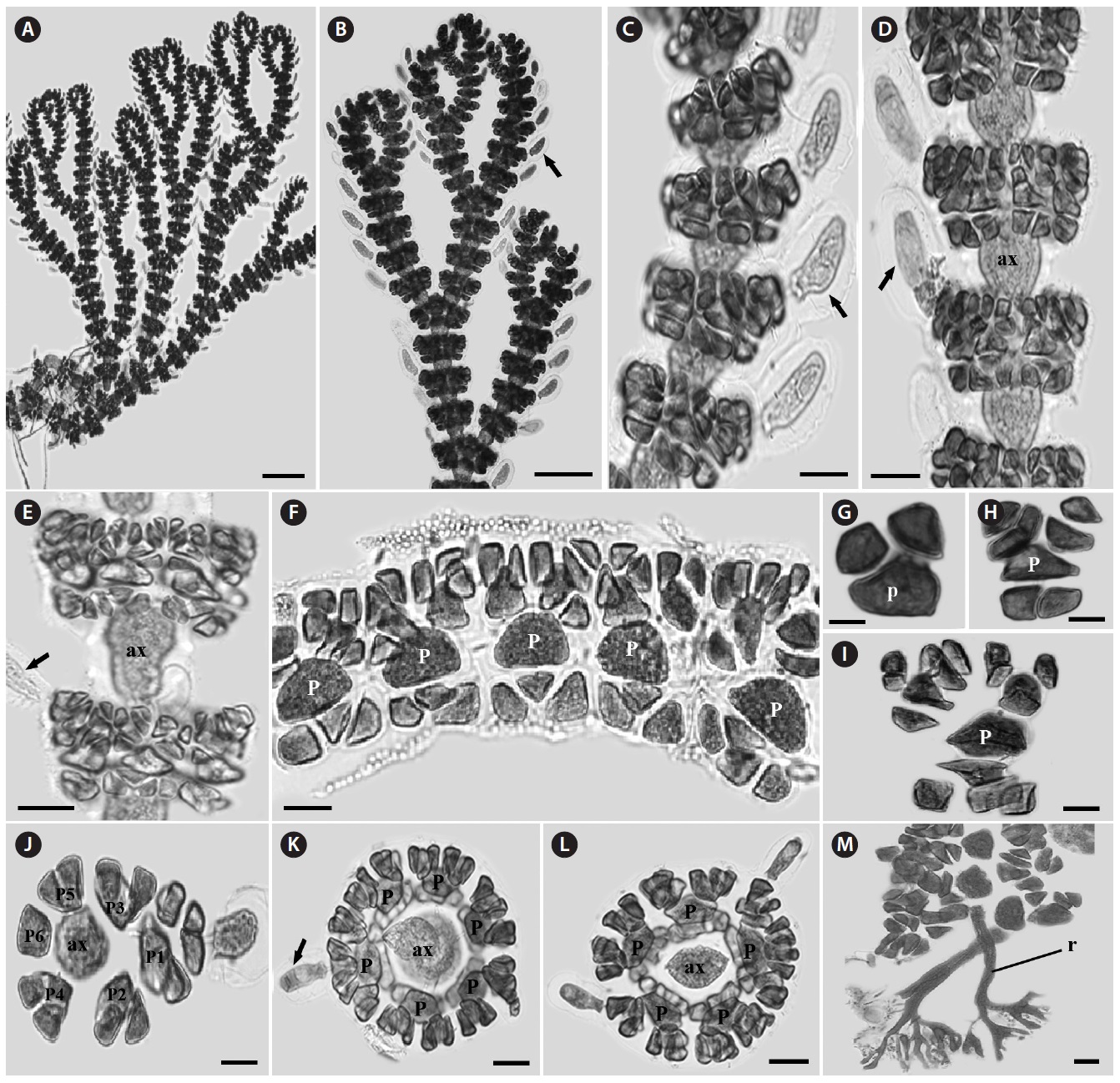
Morphology: Thalli up to 14-25 cm high, 0.1-0.2 cm wide (Fig. 2A), flat, erect, red in color, and attached to the substrate by a thick and circular holdfast (Fig. 2B). They have cartilaginous single main axis with a prominent midrib (Fig. 2C) and numerous microscopic veins, bearing series of unilaterally branches (Fig. 2F). Second-order blades usually 2-10 cm long, produced along the midrib of mother blades. Each blade has one to several bladelets by producing many higher orders of bladelets (Fig. 2D and 2E). Margin of blade serrulate and toothed (Fig. 2G and 2H). Blades monostromatic to polystromatic (Fig. 2I-2K). In tetrasporic plants, tetrasporangia developed in sori on entire blades which lack midrib cortication, derived first from the lateral pericentral cells and later from cells of the second- and third- order rows, rarely from cortical cells (Fig. 2L). Tetrasporagia mature acropetally and then outwards from the midrib, producing 4-7 tetrasporangia across the blade. Mature tetrasporangia 24.5-22.9 μm in diameter, spherical, and tetrahedral (Fig. 2M).
Remarks: This species was firstly described by Harvey (1857) as
>
Gayliella fimbriata (Setchell & N.L. Gardner) T. O. Cho & S. M. Boo. (
Korean name: 깃색동풀(신칭)
Taxonomic position:
Phylum Rhodophyta
Class Florideophyceae
Order Ceramiales
Family Ceramiaceae
Genus
Holotype: Marchant (no. 87a), UC.
Type locality: Eureka, near La Paz, Mexico.
Specimens examined: CUK6119 (Seongsan, Jeju: 23 Jul 2008), CUK6797 (Gampo, Kyeungbuk: 4 Nov 2009).
Habitat: Epiphytic on other algae.
Morphology: Thalli up to 1-2 cm high, consisting of erect and prostrate axes (Fig. 3A). Branches alternate (Fig. 3A and 3B).
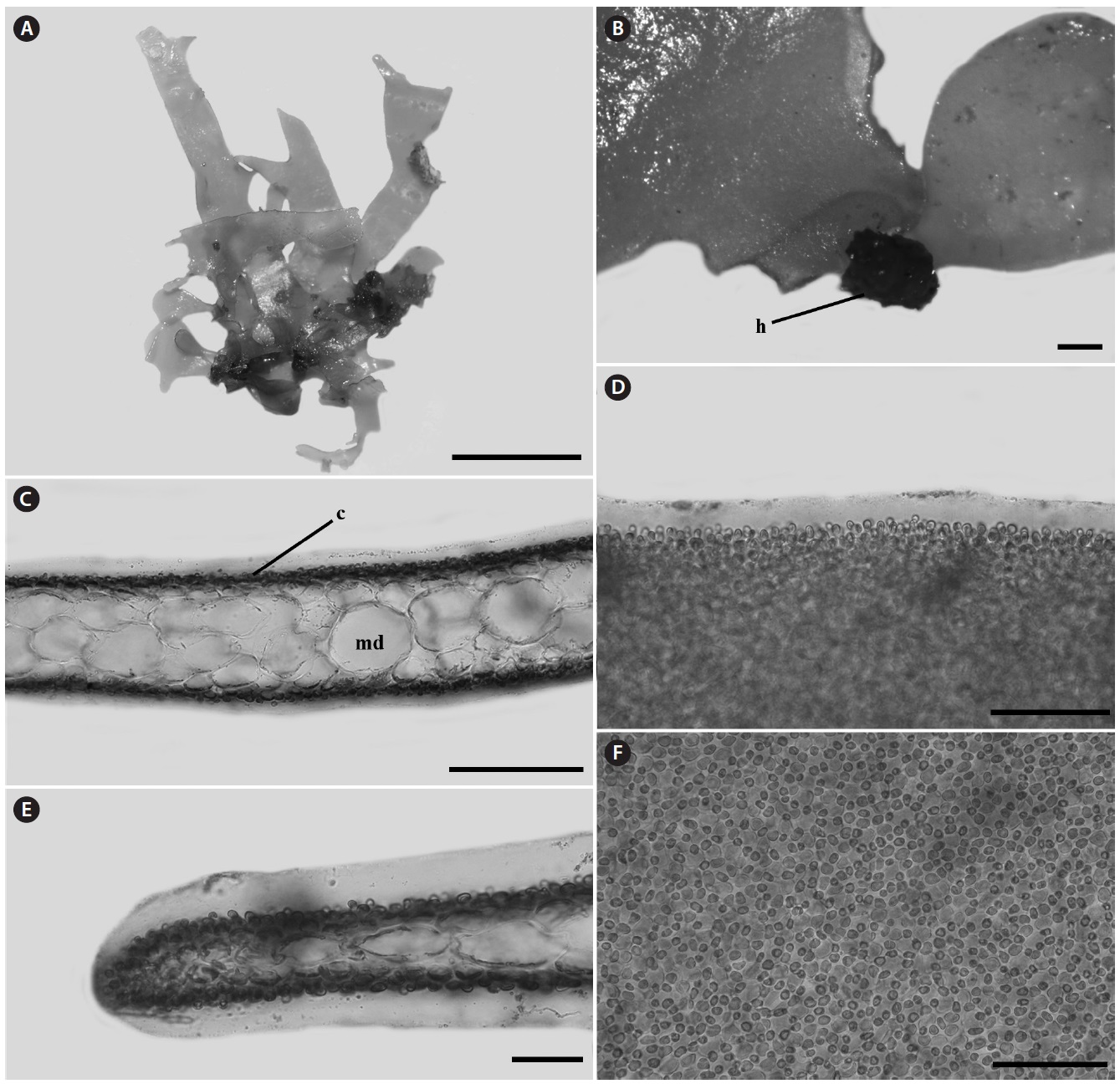
Gland cells clavate and developed from cortical cells of acropetal corticating filaments, but rarely basipetal corticating filaments (Fig. 3B-3E). Cortical band composed of three corticating filaments (Fig. 3F-3I). The third cortical initial produced horizontally from periaxial cell (Fig. 3I). Periaxial cells five to seven (Fig. 3J-3L). Rhizoids unicellular with a terminal digitate pad and produced from periaxial cells (Fig. 3M).
Remarks:
>
Leptofauchea rhodymenioides W. R. Taylor 1942 (
Korean name: 납작잎바위주걱(신칭)
Taxonomic position:
Phylum Rhodophyta
Class Rhodophyceae
Order Rhodymeniales
Family Faucheaceae
Genus
Holotype: Wm. R. Taylor (cystocarpic and tatrsporic), MICH 20253.
Type locality: Aruba Island, Netherlands West Indies.
Specimens examined: CUK9577 (Juk-do of Ulreung-gun: 23 Apr 2013).
Habitat: Epiphytic on shells.
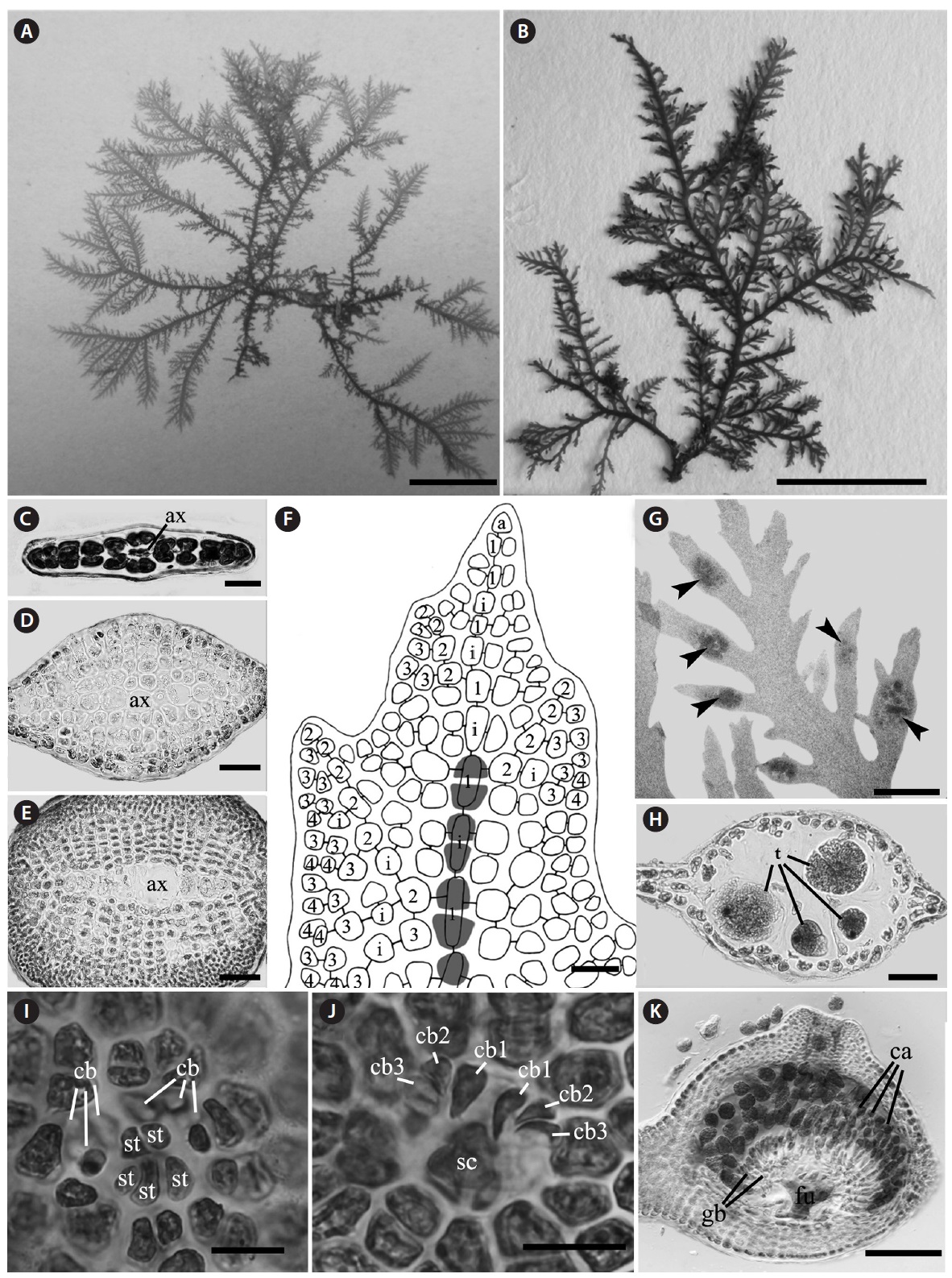
Morphology: Thalli 1-2.5 cm high, flat, membranous (Fig. 4A), gelatinous (Fig. 4D), and pink in color. They grow on the shells by small discoid holdfast (Fig. 4B). They expanding with broadly rounded apices and usually dichotomously branched in old plants, and sometimes attached to other blades (Fig. 4A). In surface view, outer cortical cells scattered around inner cortical cells, and the cortical cells loosely arranged (Fig. 4F). Thalli composed of outer cortex and inner medullar layers (Fig. 4C). Outer cortical cells small, rounded, and composed of one cell layers (Fig. 4C). Inner cortical cells rounded to elliptical and composed of 1-2 cell layers. Medullar cells large, polygonal to elliptical, thin-walled, composed of 2-3 cell layers (Fig. 4C). Margins of thallus, cortex and medulla cells close-packed (Fig. 4E). Outer cortical cells scattered around inner cortical cells, and loosely arranged.
Remarks:
>
Sorella pulchra (Yamada) Yoshida & Mikami 1991: 129, f. 1-11. (
Korean name: 가지분홍잎사촌(신칭)
Taxonomic position:
Phylum Rhodophyta
Class Florideophyceae
Order Ceramiales
Family Delesseriaceae
Genus
Holotype: SAP 048988.
Type locality: Hayama, Kanagawa Prov. Japan.
Specimens examined: JN120727-JN12072726 (Dueok-do, Wan-do: 27 Jul 2012); JN120726-1 (Jak-do, Yeosu: 26 Jul 2012).
Habitat: Epilithic on bed rock.
Morphology: Fronds 3-6 cm high and 0.6 mm wide in the broadest portion, attached to the substratum by discoid holdfast, sometimes forming creeping branch on the basal part. Short branches densely produced by alternately pinnate manner from margins of axial branch in one plane and some of them elongated. The elongated branches produce short branchlets again in the same manners (Fig. 5A and 5B). Fronds cylindrical in the basal part and gradually compressed toward the top of branch having acute apex (Fig. 5C-5E). In the cross-section view of the middle and lower parts of branches, uniserial cells involving an axial cell surrounded by several cortical cell layers. Cortical layers decreasing toward the apical part of branch forming mostly polystromatic frond except apical parts (Fig. 5C-5F). The midrib and microscopic veins inconspicuous. The growth of thalli derived by transverse division of apical cell forming primary cell row, followed longitudinally for secondary cell rows. The secondary cell rows reach the thallus margin, and intercalary cell divisions in each cell row. Some cells in the secondary cell rows cutting off third-order cell rows abaxially (Fig. 5F).
Tetrasporangial sori mostly formed on the upper portion of branchlets and there swollen in both sides of branchlets (Fig. 5G and 5H). The sori consist of two tetrasporangia layers. Mature tetrasporangia 50-60 μm in diameter, spherical and divided tetrahedrally (Fig. 5H). Procarps scattered in the apical parts of the branches and composed of a supporting cell, a group of sterile cells, and two groups of four-celled carpogonial branches (Fig. 5I and 5J). Mature cystocarps 450-550 μm in diameter and composed of a large branched fusion cell, shortly radiate gonimoblast filaments from the fusion cell, two or three
celled chains of carposporangia on the terminal of each gonimoblasts filament, and 5 to 9 cells thick ostiolate pericarp. Cystocarps hemispherical and more swollen on one side of branches (Fig. 5K).
Remarks: This species was firstly described by Yamada (1938) based on the tetrasporophytes and named
Recently, we collected this species from two islands (Jak-do, Yeosu and Dueok-do, Wando) on the southern coast of Korea. These specimens were growing on bedrock at 5~15m depth of the subtidal zone. Our morphological observations are well agreed with the description of Yamada (1938) and Yoshida and Mikami (1991). Therefore, we present