Microbial fuel cell (MFC) is a device that uses electrically-catalytic microorganisms to convert organic or inorganic substances into electricity [1,2]. Increasing interest has been given to the MFC due to its potential of generating alternative energy using organics contained in the wastewater. Therefore, MFC has been introduced as a good candidate for the sustainable and renewable energy production in the future. However, its field application has rarely been reported because of low power generation efficiency and high operating cost [3]. Therefore, a number of previous studies have focused on how to increase the electric power density produced from MFCs.
Microorganisms obtain electrons by oxidizing organic substances and then transfer the electrons eventually to extracellular electron acceptors via their metabolic process [4]. In the MFC system, electrically-catalytic microorganisms transfer electrons to electrode as a final electron acceptor [5]. Various mechanisms that explain the electron transfer from bacteria to the anodic electrode have been suggested: 1) electron transfer via direct contact between cells and electrode through multiheme cytochromes in the outer cell membrane [6]; 2) electron transfer using soluble mediators that function as electron shuttles from cells to electrode [7,8]; and 3) electron transfer through extracellular appendages referred to as bacterial nanowires or nano-pili [9-11]. Anode plays an important role in transferring electrons within the circuit of MFC, and it serves as a medium on which the electrically-catalytic microorganisms are attached. In recent years, several studies demonstrated that microbial communities have the capability to transfer electrons directly to electrode without mediators (mediator-less MFC) using anode electrode as the sole electron acceptor [12,13]. Electrogenic bacteria such as
In this study,
2.1. Bacterial Strain and Culture Conditions
2.2. Microbial Fuel Cell Operation
A dual-chamber MFC reactor made of acrylic with a working volume of 150 mL was employed in this study. The anode and cathode compartments were separated by a Nafion 117 proton exchange membrane (PEM; DuPont, Wilmington, DE, USA). Prior to use, PEM was pre-treated by boiling in H2O2 (30%, v/v) and deionized water, followed by soaking in 0.5-M H2SO4 and then deionized water, each for 1 hr as described previously [15,16]. The cathode chamber was filled with an electrolyte solution containing 30-mM Tris buffer solution (pH 7.0) [17,18], which was continuously purged with water-saturated air. The pure culture of
[Table 1.] Performance of microbial fuel cell operation in this study
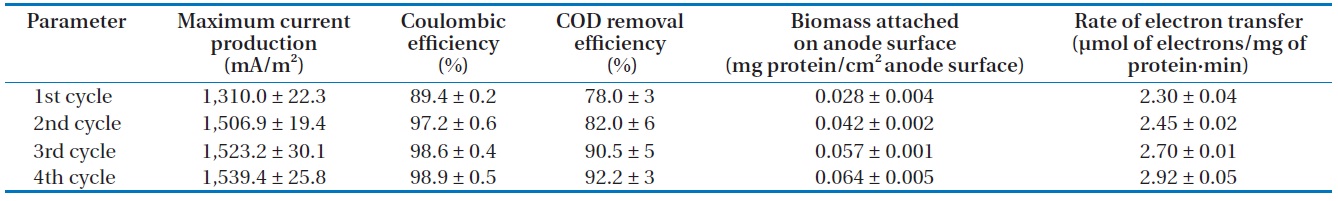
Performance of microbial fuel cell operation in this study
Quantity of microbial biofilm was measured by its protein concentration at the end of batch operation. The protein extraction procedure was described in a previous study [6]. Total protein concentration was determined by Bradford analysis with bovine serum albumin as a standard (Quick Start Bradford protein assay; Bio-Rad Company, Hercules, CA, USA). Aqueous samples were collected from the anode chamber and their organic levels were analyzed as chemical oxygen demand (COD) as described by Standard Methods [19]. The recorded voltage was converted into current density using Ohm’s law (
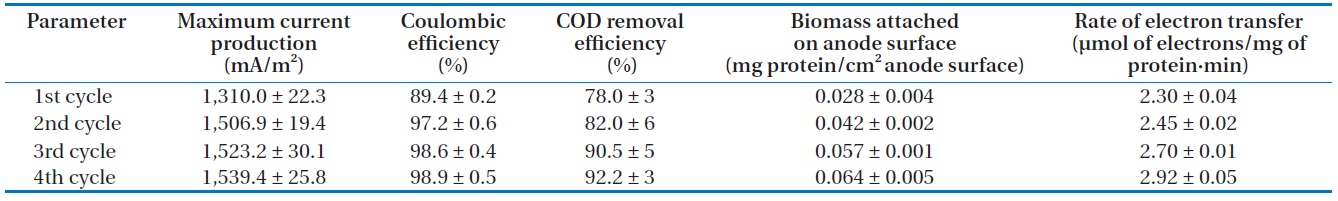
0.03 mA, the medium in the anode chamber was replaced with a fresh lactate medium. Then, current production rapidly returned to a maximum level and the maximum current production was sustained for a couple of days. Note that as the replacement of medium was repeated, the time to reach the maximum current production became shorter and the level of maximum current production was increased. The levels of maximum current production in the 2nd, 3rd, and 4th cycles were 1,506.9 ± 19.4, 1,523.2 ± 30.1, and 1,539.4 ± 25.8 mA/m2 (n = 3), respectively (Table 1). The pattern of immediate resumption in current production was very similar in 4 times of exchange of anodic medium. This indicated that microorganisms attached to the anode were primarily responsible for the current production [6,12,22]. A more detailed discussion is provided in the following sections with the results obtained from the monitoring of the MFC operation parameters, including the concentration of biomass attached on the anode surface, electron recovery by lactate oxidation, and COD removal efficiency.
The concentration of COD was monitored over the course of MFC operation. In the anode chamber, the COD removal efficiency was increased from the first cycle (78.0% ± 3%, n = 3) to the final cycle (92.2% ± 3%) (Table 1). The COD removal efficiency clearly demonstrated the feasibility of removing organic carbon using MFC technologies. Assuming that lactate are completely oxidized to carbon dioxide, the recovery of electrons from the substrate oxidation was calculated by comparing the total charge or number of coulombs through the MFC circuit (Fig. 1) with the theoretical value from substrate oxidation. The total electron recovery is a function of the substrate concentration for lactate-fed MFC. Values of CE or electron recovery for the lactate oxidation were 89.4% ± 0.2%, 97.2% ± 0.6%, 98.6% ± 0.4%, and 98.9% ± 0.5% (n = 3) for the 1st, 2nd, 3rd, and 4th cycles of MFC run, respectively (Table 1). By stoichiometry, oxidation of lactate to carbon dioxide yields 12 electrons:
The CE in our study was relatively higher than those obtained in previous studies [12,20,23]. Given that current was produced in the mediator-less MFC, the generated electrons were transferred directly to electrode, not to soluble electron mediators such as sulfate [20]. Based on these results, it is clear that
Lactate was consumed at the expense of current production and cell growth, indicated by the increase in cell biomass over time in the anode chamber (Table 1). Cells continuously formed dense biofilm on the anode surface and the biomass reached 0.028 ± 0.004 mg protein/cm2 anode surface (n = 3) at the beginning of MFC operation. It increased to 0.064 ± 0.005 mg protein/cm2 anode surface at the end of the MFC operation, which was higher than the values observed in prior studies [6,12]. Rate of current production calculated based on the attached bacterial populations on the anode surface was increased from the first (2.30 ± 0.04 µmol of electrons/mg of protein·min, n = 3) to final (2.92 ± 0.05 µmol of electrons/mg of protein·min) cycles of MFC run (Table 1), indicating that electron transfer to electrode was increased. In this study, the rate of electron transfer to electrode was higher than those reported by Liu et al. [21]. They reported that the electron transfer rates using flat gold and carbon cloth as electrodes were 2.2 and 2.4 µmol of electrons/mg of protein·min, respectively. Thus, it can be concluded in this study that the current production efficiency was related to the biomass of biofilm formed on the electrode, which was increased as the MFC run was repeated.
3.3. Biofilm Formation on Anode
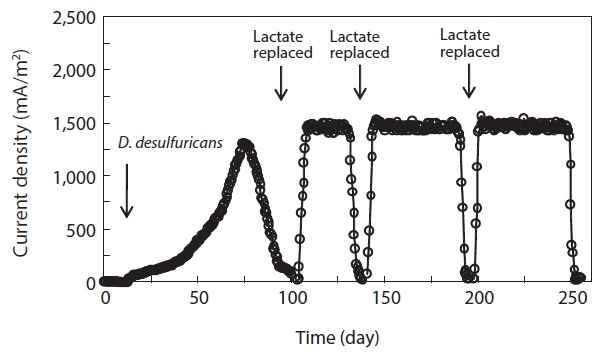
At the end of batch operation, the electrode was collected from the anode chamber. Biofilm formed on the electrode surface in each cycle of MFC operation was examined by SEM (Fig. 2). The morphology of bacteria was rod-shaped. Biofilm was formed on the electrode surface in the first cycle of MFC run, which was referred to as the primary biofilm in Fig. 2(a). During further batch operation, significantly thick and dense biofilm was formed on the electrode, referred to as the secondary, tertiary, and quaternary biofilm in Fig. 2(b)-(d). The formation of the primary biofilm resulted in a gradual increase in current production. When further biofilm was formed, the current production was more rapid and the maximum current production levels were higher than that obtained from the first cycle. These results indicated that
In this study, we found that