The family Labridae in the order Perciformes comprises 453 species and 68 genera worldwide (Nelson, 2006), with 20 species and 14 genera in Korea (Kim et al., 2005). This family is one of the most diverse groups of marine fishes, displaying various shapes, colors, and sizes (Nelson, 2006). Among them, the genus Stethojulis has a wide extended range from the Red Sea to Hawaii; it consists of 10 species worldwide (Randall, 2000), although only one species, S. interrupta, occurs in Korea. A juvenile color pattern that differs from that of the adult is present in some fish groups (Allen, 1991), and juveniles in most of the family Labridae differ in their color patterns from the adult (Mahon, 1994; Victor et al., 2001; Westneat, 2002). Thus, recent studies of juvenile identification have used both morphological descriptions and molecular methods (Chow et al., 2003; Li et al., 2006; Robertson et al., 2007; Victor et al., 2009; Kwun et al., 2012). In this study, we confirmed that a single specimen collected from Ulsan, Korea, was Stethojulis trilineata using molecular analyses, and we describe the species as the first record based on a specimen.
A single specimen of Stethojulis trilineata was collected from Ulsan, Korea on October 26, 2012, and was fixed in 99% ethanol. Counts and measurements followed Hubbs and Lagler (2004), using a vernier caliper to the nearest 0.1 mm. The vertebrae were counted from a radiograph (Softex HA-100; Softex, Tokyo, Japan). The specimen was deposited at the National Institute of Biological Resources (NIBR), Korea. Molecular identification of this specimen was performed using VF2 (5′-TCAACCAACCACAAAGACATTGGCAC-3′) and FishR2(5′-ACTTCAGGGTGACCGAAGAATCAGAA-3′) primers, which amplify the mitochondrial DNA cytochrome oxidase subunit I (COI) (Ward et al., 2005; Ivanova et al., 2007). Genomic DNA was extracted from eye using Chelex 100 resin (Bio-Rad, Hercules, CA, USA). A polymerase chain reaction (PCR) was performed in a total volume 50 μL containing DNA template 5 μL, dNTP 4 μL, 10x buffer 5 μL, Taq polymerase 0.5 μL, reverse primer 1 μL, forward primer 1 μL, and distilled water. PCR was conducted under the following conditions: initial denaturation for 1 min at 95℃, followed by 35 cycles of 1 min at 95℃ for denaturation, 1 min at 50℃ for annealing, and 1 min at 72℃ for extension, with a final extension at 72℃ for 5min. The PCR products were purified using ExoSAP-IT (United States Biochemical Corp., Cleveland, OH, USA). The DNA was sequenced using an ABI 3730XL sequencer and an ABI PRISM BigDye Terminator (ver. 3.0) Ready Reaction Cycle Sequencing Kit (Applied Biosystems, Foster City, USA). The nucleotide sequence was deposited in the DDBJ/EMBL/GenBank database (accession number: KF264566). The sequence was aligned with ClustalW (Thompson et al., 1994) in BioEdit (ver. 7) (Hall, 1999). The sequences of six Stethojulis species (S. albovittata, S.interrupta, S. trilineata, S. strigiventer, S. bandanensis, and S. balteata), from the National Center for Biological Information database were used for the sequence comparison. Genetic distances were calculated with the Kimura-2-parameter model (Kimura, 1980) in MEGA 5 (Tamura et al., 2011).
Labrus trilineata Bloch and Schneider, 1801: 253 (type locality: Corimgo, Coromandel, India).
Julis sebanus Valenciennes in Cuvier and Valenciennes, 1839: 474 (no locality).
Julis (Halichoeres) phekadopleura Bleeker, 1849: 8 (type locality: Boleling, Bali).
Halichoeres sebae Kner, 1860: 50 (no locality).
Stethojulis phekadopleura Bleeker, 1862: 134 (type locality: Indonesia).
Stethojulis filholi Sauvage, 1880: 225 (type locality: Fiji).
Stethojulis trilineata: Bleeker, 1862: 131 (India); Günther, 1862: 140 (East Indian Archipelago); Masuda et al., 1984: 207 (Tokara Island and the Red Sea); Randall, 2000: 36 (Indo-South Pacific); Hutchins, 2001: 269 (Western Australia); Shimada, 2002: 984 (Japan); Adrim et al., 2004: 125 (Anambas, South China Sea); Motomura et al., 2010: 172 (Japan); Allen and Erdmann, 2012: 720 (Indo-Pacific).
NIBR-P0000020417, 1 specimen, 23.6 mm in standard length (SL), Ulsan, Nasa-ri, 26 Oct, 2012, collected by Kwun
HJ and Jang IC.
Proportion as % SL: body depth 22.5; body width 10.6; head length 31.8; snout length 8.5; eye diameter 8.1; interorbital width 13.6; upper jaw length 4.2; predorsal length 32.6; prepelvic length 32.6; preanal length 55.5; base of dorsal spine length 17.8; base of dorsal ray length 25.8; pectoral fin length 14.4; pelvic fin length 11.0; caudal peduncle depth 12.3; caudal peduncle length 10.6.
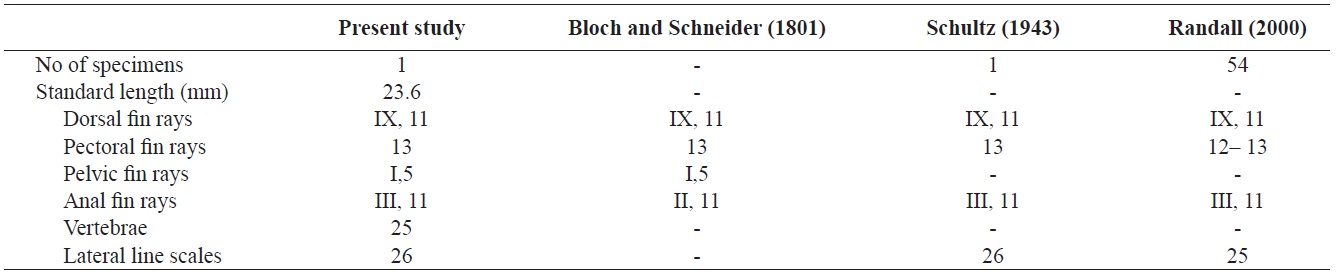
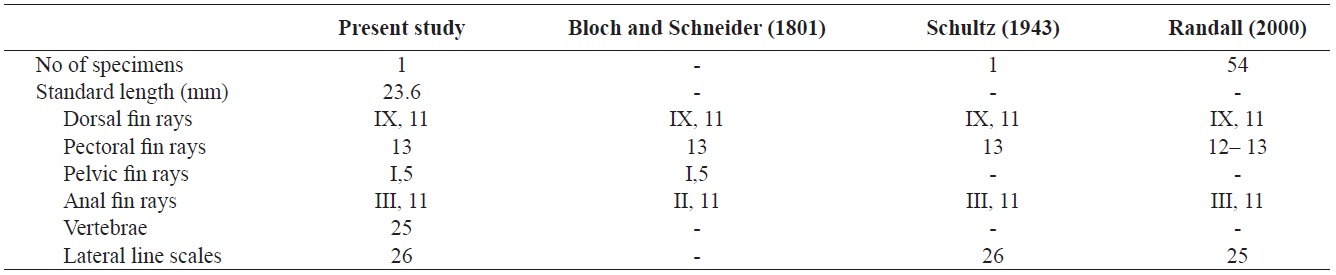
Counts are shown in Table 1. Body elongated, compressed, and moderately deep. Mouth small, terminal, and lips thin. The anterior ends of both jaws coincide well. A single row of small conical teeth on both jaws and the two front teeth are more developed than the others. Posterior tip of maxilla not reaching to the anterior margin of the eye. Dorsal margin of the head slightly sloping and single pair of nostrils located in front of the eyes. Body covered with large cycloid scales but head scaleless. Lateral line continuous, but tending sharply downward below the 8th dorsal fin ray to the straight caudal fin base. Anus located in middle of the body. Opercle large, and posterior margin of opercle beyond the origin of pectoral fin. Margin of opercle smooth, but with two small spines. Dorsal fin with no obvious notch between spinous and soft portions. Pectoral fins somewhat large and fan-shaped. Pelvic fin short and origin of the pelvic fin below posterior end of the base of the pectoral fin. Caudal fin slightly rounded.
When fresh, the body and head are yellowish-green dorsally and pale green ventrally. A silver–white stripe extends from the front of the snout through the eye to the caudal peduncle, fading posteriorly. All fins are transparent, and dark spots occur between the 10th and 11th dorsal fin rays and caudal peduncle region. After fixation, the body and head are pale green, fading gradually ventrally. The body and head, except for the ventral part of the head and abdomen, have many small melanophores. Posterior to the opercle and on the cheek below the lower jaw are small dark spots. A stripe with small melanophores extends from the snout to the caudal peduncle. Small melanophores occur along the dorsal fin, pectoral fin, and caudal fin rays.
Stethojulis trilineata is distributed in the Indo-Pacific (Randall, 2000), South China Sea (Adrim, 2004), Japan (Masuda et al., 1984; Shimada, 2002), and Korea (southern part of the East Sea, Ulsan; present study).
The specimen collected from Ulsan belongs to the genus Stethojulis in having a continuous spinous and soft portion of the dorsal fin, a lateral line sharply bent but connected, a naked head, and XI dorsal fin spines (Table 1, Fig. 1). Compared with the original description, this specimen corresponds to S. trilineata in having a green body, scaleless head, XI dorsal fin spines, and 13 pectoral fin rays (Table 1). Based on comparisons with juveniles of S. trilineata reported by Randall (2000), the specimen (juvenile) in this study is similar in having dark spots between the 10th and 11th dorsal fin rays and a caudal peduncle region, and with small melanophores behind the opercle. To identify the species exactly, we analyzed the mitochondrial DNA COI gene, which indicated that the specimen’s DNA sequence almost corresponded to that of S. trilineata (d = 0.002–0.005) and differed slightly from that of S. interrupta (d = 0.020–0.023). Thus, this specimen was identified as S. trilineata based on molecular and morphological analyses. Although S. trilineata is morphologically similar to S. interrupta (also reported in Korea) in the number of its dorsal fin rays (S. trilineata IX, 11 vs. S. interrupta IX, 11–12) and pectoral fin rays (12–13 vs. 13, respectively) (Randall, 1986, 2000; Shimada, 2002; Kim et al., 2005; Allen and Erdmann, 2012), differences exist in the numbers of gill rakers (25–28 vs. 19–23, respectively), suborbital pores (8–12 vs. 6–9, respectively), dark spots in the caudal peduncle region (present only in females vs. absent in both males and females, respectively), and the last stripe starting from the snout (extends to the caudal peduncle vs interrupted on the anterior half of the body) (Westneat, 1999; Randall, 2000; Shimada, 2002; Allen and Erdmann, 2012). The juvenile of S. trilineata is also similar to that of S. interrupta in having a small dark spot posteriorly on the dorsal and anal fins near their bases, but the former is clearly distinguishable from the latter in having a narrow silver–white stripe extending from the frontof the snout to the caudal fin (vs. two narrow black stripes). Similarly, the juvenile of S. trilineata has a silver–white stripe, and not only has dark spots between the 10th and 11th dorsal fin rays and the caudal peduncle region, but also small dark spots on the posterior region of the opercle. These characteristics differ from those of juvenile S. strigiventer, which has a narrow white stripe from the front of the snout through the upper part of the eye and continuing to the upper caudal fin base, and a more irregular white stripe from the corner of the mouth through the lower part of the eye to the lower caudal fin base; S. albovittata, which has a small black spot near the base of the last membrane of the dorsal and anal fins; and S. balteata, which has two small spots at the base of the caudal fin, a smaller black spot midlaterally on the caudal peduncle, and a black spot rimmed with yellow posteriorly on the dorsal and anal fins (Randall and Kay, 1974; Randall, 2000).
The family Labridae is one of the groups in which the color patterns of juveniles differ from the adult patterns, and members also display sexual dimorphism (Mahon, 1994; Victor et al., 2001; Westneat, 2002). Stethojulis trilineata also has dark spots on the dorsal fin rays and caudal peduncle, which disappear gradually as it grows, when three narrow stripes appear. Thus, further study is required to provide an exact morphological description of adult S. trilineata. We propose the new Korean name “Se-jul-mu-ji-gae-nol-rae-gi” for S. trilineata, based on its morphological characteristic of three narrow longitudinal rows on the body of the adult.