We investigated the toxicological effects of Aroclor 1254 on the fertilized eggs, embryos and larvae of the olive flounder Paralichthys olivaceus. The survival rate and hatching success of the embryos decreased significantly in treated groups in an Aroclor 1254-dose-dependent manner. Significant differences were found at ≥5 µg/L Aroclor 1254 compared to the control group. Hatching success occurred at ≤10 µg/L Aroclor 1254, which was not significantly different to the control. Embryo malformation increased significantly at ≥1 µg/L, and included yolk-sac and tail-flexure abnormalities. There was a significant decrease in the survival rate of the larvae at ≥5 µg/L, which was accompanied by the malformations described above. Notably, concentrations as low as 1 µg/L caused a significant increase in abnormalities in the larvae, including incidences of multi-focal hemorrhages, pericardial and yolk-sac edema, inhibition of swim bladder inflation and severe developmental delay. The responses to Aroclor 1254-induced toxicity were generally similar among fertilized eggs, embryos and larvae from three separate flounder hatcheries: Cheju Island, Yeosu and Chungnam, South Korea. These results indicate the high acute toxicity of Arolcor 1254 concentrations of which as low as 1 μg/L in olive flounder larvae can affect unhatched embryos. To conclude, the average LC50 values for Aroclor 1254 in the embryos and larvae were 50.92 and 3.08 μg/L, respectively. Additionally, the average EC50 values, based on the rate of damage were 14.72 and 5.61 μg/L, respectively.
Polychlorinated biphenyls (PCBs) are considered to be important environmental pollutants worldwide, despite their use being restricted in most countries since the 1970s. Due to their high persistence and strong lipophilic properties, PCBs have bioaccumulated in aquatic food webs. PCB mixtures have very low solubility in water but high solubility in oils and low-polarity organic solvents. Even though the water solubility of Aroclor 1254 is only 2.7 μg/L, PCBs are classed as probable human carcinogens by the Environmental Protection Agency (EPA), based on substantial evidence of cancer in animals (Walker et al., 1994). Despite the decrease in PCB levels during the last 30 years, they remain a major contaminant in fish (Bignert et al, 1998).
The early life-stage toxicity test is generally considered by aquatic toxicologists to be the most useful test for risk assessment. The embryonic period begins with fertilization or union of the gametes, and is characterized by an endogenous food source (yolk), finally ending at hatching. The larval period begins with egg hatching and lasts until the appearance of a full complement of fin rays and spines. This period is divided into two phases; the initial yolk-sac larva or alevin period, which begins at hatching and ends after complete absorption of the yolk, resulting in juvenile (Jones et al., 1978).
Aroclor 1254 is a PCB mixture that is acutely toxic to aquatic animals (Hansen et al., 1971; Schimmel et al., 1974). Mac et al. (1993) reported a strong correlation between total egg mortality and total PCB concentration in the eggs. It has been suggested that PCBs in the Great Lakes (USA) were responsible for the significant mortality of the early life stages of the lake trout,
Previous studies have investigated the ecotoxicological responses of fish from a single location. Such narrow studies cannot account for geographical or physiological patterns of adaptation that can occur in the natural environment (Hallare et al., 2005). For example, in South Korea, different fish stocks are used at different aquaculture facilities, including Cheju Island, Yeosu, and Chungnam. The condition of the mother fish differs according to the aquaculture farm she comes from and hence, the quality of eggs received in the laboratory differs, depending on where they are sourced. Accordingly, this study compared the responses of the olive flounder from different regions of Korea, as this has important implications for monitoring environmental conditions using the olive flounder as an indictor species. The main purpose of this study was to investigate the effects of sub-lethal Aroclor 1254 exposure on survival and hatching, as well as on embryo and larvae deformities and survival of the olive flounder.
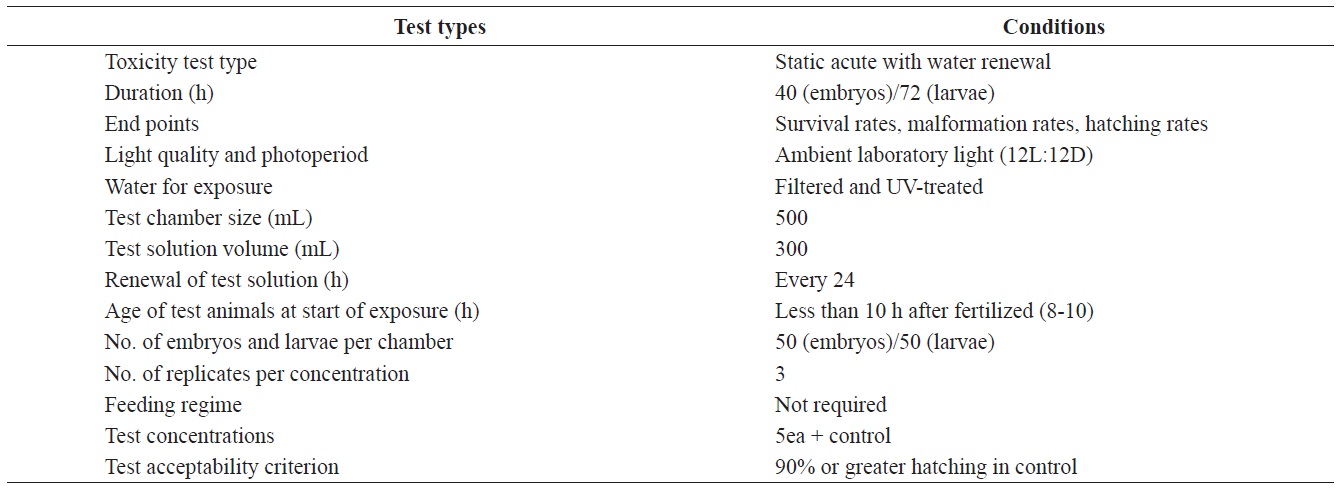
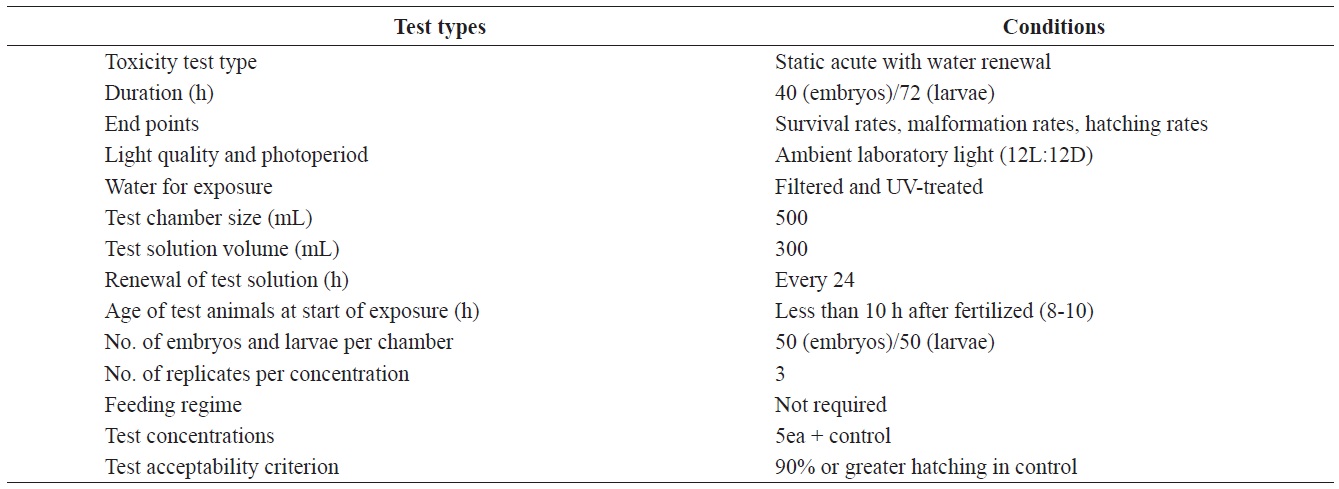
Test conditions for the acute toxicity tests with olive flounder Paralichthys olivaceus embryos and larvae
>
Experimental animals and water conditions
Fertilized olive flounder eggs were obtained from hatcheries in Cheju Island, Yeosu and Chungnam, South Korea. The water quality parameters measured for the bioassays conducted were as follows; pH, 8.10 ± 0.2; salinity, 32.70 ± 0.4 ‰; dissolved oxygen, 6.74 ± 0.84 mg/L; chemical oxygen demand, 1.52 ± 0.008 mg/L. All experiments were carried out separately for each region, but at a single test location. All experiments were conducted at a seawater temperature of 20 ± 0.5℃ under a 12-h light/ 12-h dark cycle, over either a 36-h or 72-h period. Other pertinent test conditions for the acute toxicity tests are summarized in Table 1.
Aroclor 1254 was purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Five solutions (1, 5, 10, 20, and 40 μg/L) were prepared by diluting the stock solution. The stock solution was made up in seawater that had been filtered through a grade GF/C, Whatman filter (Maidstone, UK) and ultraviolet-treated.
The eggs obtained from each regional hatchery were approximately at the 8-10 h post-fertilization (blastula) stage. They were transferred directly into cleaned 500 mL glass beakers containing 1, 5, 10, 20, or 40 μg/L Aroclor 1254 for static exposure. Each Aroclor 1254 concentration (containing 50 embryos), and the controls were carried out in triplicate. Embryonic development was monitored every 3 h, up to an endpoint at 40 h, or until hatching was complete. The beaker contents were replaced every 24 h throughout the toxicity test (Table 1). At each time interval, embryo mortality, hatching success and abnormalities were noted using a microscope (×400) connected to a camera. Embryos were considered dead when any part of the embryo turned opaque or white. Preliminary Aroclor 1254 exposure experiments identified embryonic malformations such as irregularities in the egg membrane (chorion), yolk sac atrophy, and abnormal tail flexure.
Newly hatched larvae were exposed to identical Aroclor 1254 or to control conditions (Table 1). The larvae were monitored every 6 h for up to 72 h. Larvae were considered dead when there was no visible heartbeat or body movement. The survival rate was recorded throughout the experiment and abnormalities were quantified at the end of the experiment. The specific abnormalities monitored included yolk-sac edema or deformation, erosion of the fins, spinal cord curvature, and abnormal tail flexure.
Percentage data were log transformed to approximate a normal distribution, and treatment groups or hatchery locations were compared to one another using a one- or two-way analysis of variance (ANOVA). If the ANOVA was significant (
>
Embryo toxicity of Aroclor 1254
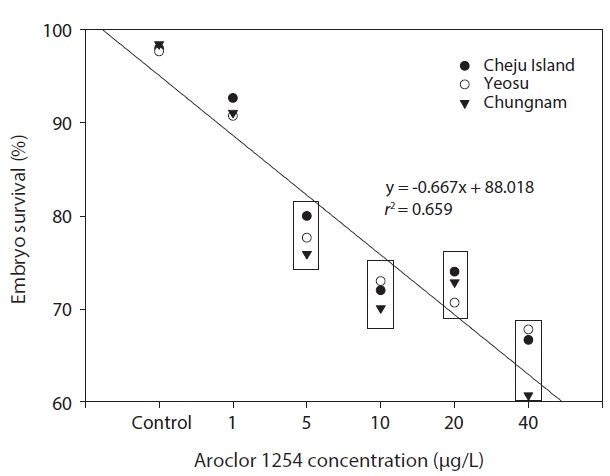
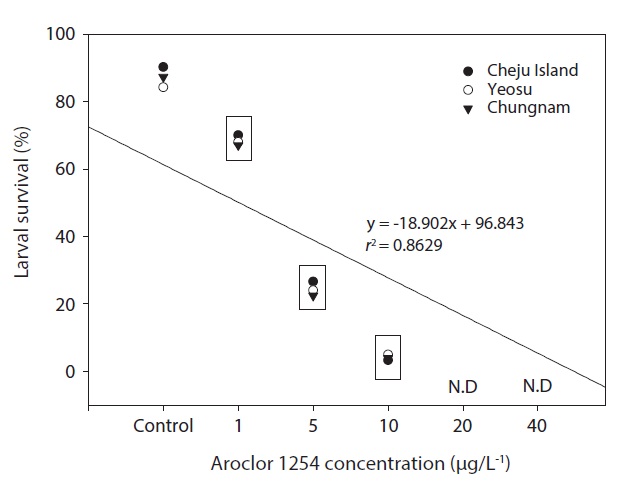
In the seawater controls and for each Aroclor 1254 concentration, survival of olive flounder embryos from all three Korean hatcheries was generally similar. Specifically, survival was above 90% in the controls and in the 1 μg/L groups, but was significantly lower in the higher Aroclor 1254 concentration groups. The combined results from the three sites suggested a linear relationship between embryo survival and Aroclor 1254 concentration (Fig. 1), with 50.92 μg/L (upper, 85.66; lower, 37.71) being the predicted concentration for 50% mortality (LC50). The results from this study confirm that the early life stages of the olive flounder are sensitive to low concentrations of Aroclor 1254; exposure reduces the survival of embryos and negatively affects their development (Fig. 1). However, the responses were generally similar in fish raised form stocks form different regions of Korea (P>0.05). This lack of variability among populations may reflect their migratory patterns and widespread distribution. These results suggest that the
olive flounder is a good indicator species for monitoring environmental conditions.
In the flag fish,
Our results showed no significant difference (
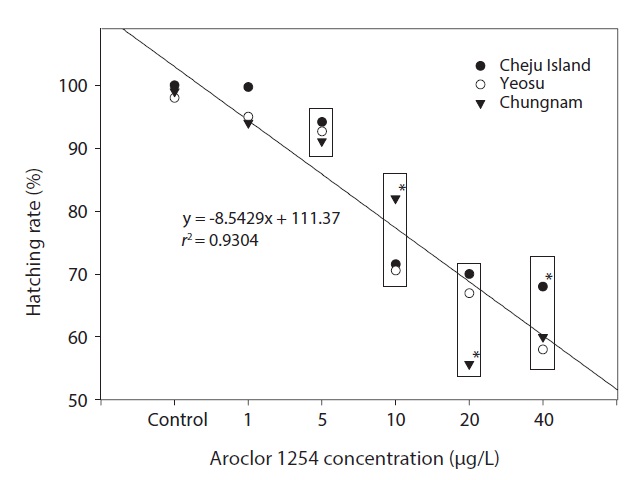
Hatching rates for embryos from all three locations were similar, especially for embryos in the seawater controls and Aroclor 1254 concentrations of ≤5 μg/L (Fig. 3). The combined results calculated using mean values showed a linear relationship: hatching rate reduced significantly with increasing
Aroclor 1254 concentrations (Fig. 3). As a comparison, the egg hatching rate of the fathead minnow,
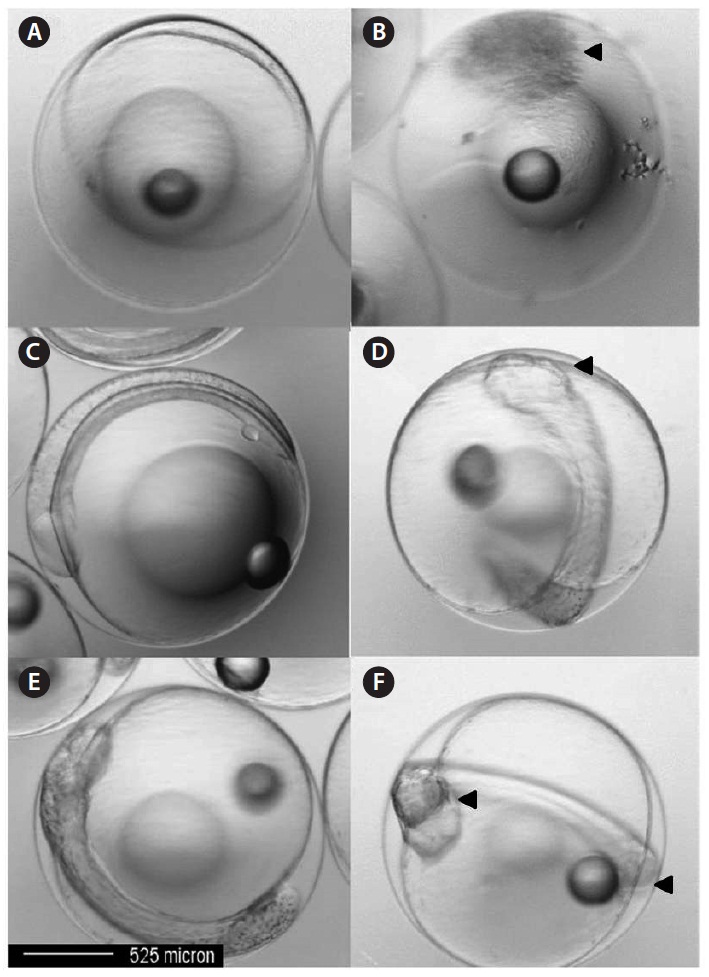
In this study, control embryos 10 h post-fertilization were normal (Fig. 4A). However, eggs exposed to Aroclor 1254 exhibited developmental abnormalities, including blastoderm coverage of only half the yolk sphere, with a darkened outer layer and an irregular outer circle (Fig. 4B). At 20 h post-fertilization, control embryos in seawater developed normally with the usual optic cup development (Fig. 4C). In contrast, 40 μg/L Aroclor 1254-treated embryos had either no or abnormal optic cup formation (Fig. 4D). At 30 h post-fertilization, skin melanophores and an eye lens appeared in the control embryos (Fig. 4E), but these features were absent in the 40 μg/L Aroclor 1254-treated embryos (Fig. 4F). Other apparent defects resulting from Aroclor 1254 exposure were an abnormal tail flexure and a morphologically irregular or atrophied yolk-sac (Fig. 4F). In this study, the most common morphological defects observed in the olive flounder embryos following Aroclor 1254 exposure were edema (atrophy) and hemorrhage in the yolk-sac (Fig. 4). The lake trout had similar malformations in response to other organic and inorganic pollutants, which are characterized by leakiness of the endothelial vessels supplying the yolk sac as a result of cardiovascular dysfunction (Guiney et al., 1990).
>
Larvae toxicity of Aroclor 1254
Survival of olive flounder larvae decreased significantly with increasing Aroclor 1254 concentration (Fig. 5); the LC50 value was 3.08 μg/L (upper, 3.38; lower, 2.78). Other studies have shown that PCB congeners in particular cause early life stage mortality in other fish species, where 3.7 and 8.7 μg/g HXCB in the Chinook salmon
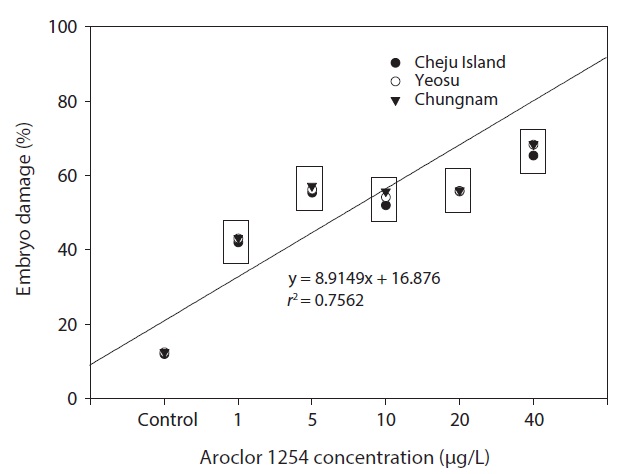
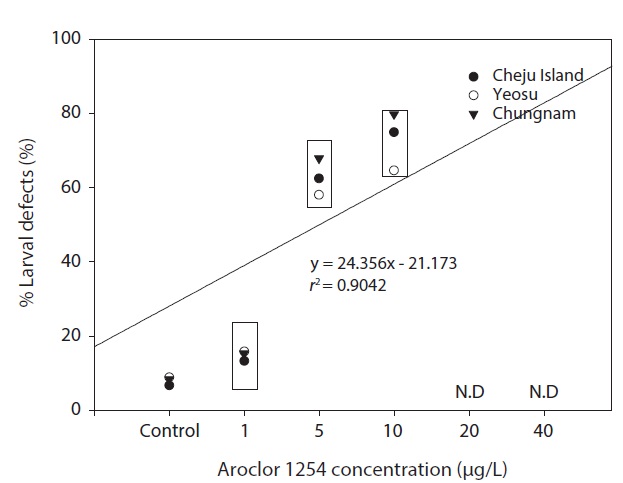
In our study, the percentage of larvae displaying developmental defects was similar in embryos collected from all three sites (Fig. 6). There were no larval abnormalities in the controls. The malformations observed can be classed as relatively unspecific responses to a number of pollutants (Strmac and Braunbeck, 1999). The percentage of larval abnormalities increased in accordance with Aroclor 1254 concentration (Fig. 6) and the Aroclor 1254 EC50 was 5.61 μg/L (upper, 17.08; lower, 4.41).
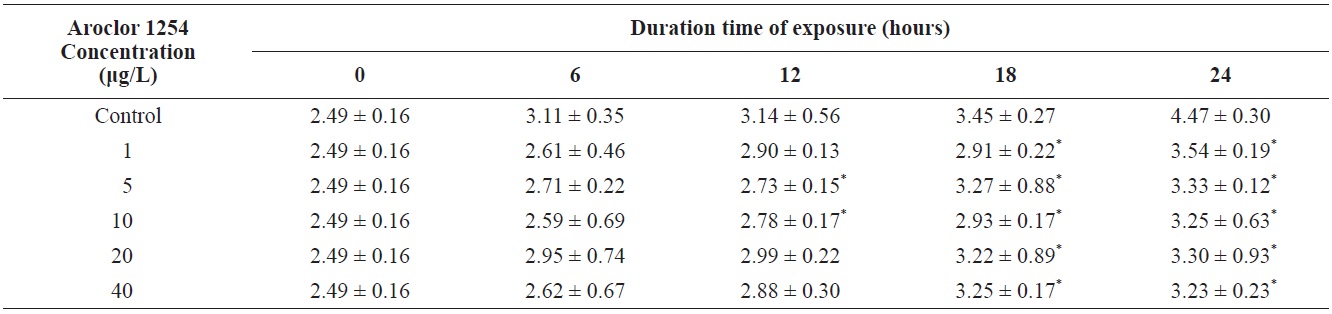
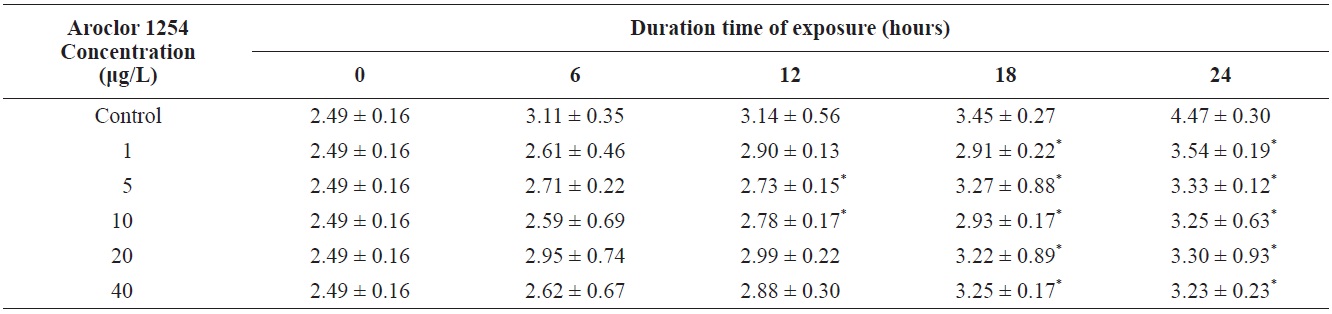
Total length of olive flounder Paralichthys olivaceus larvae exposed to various Aroclor 1254 concentrations
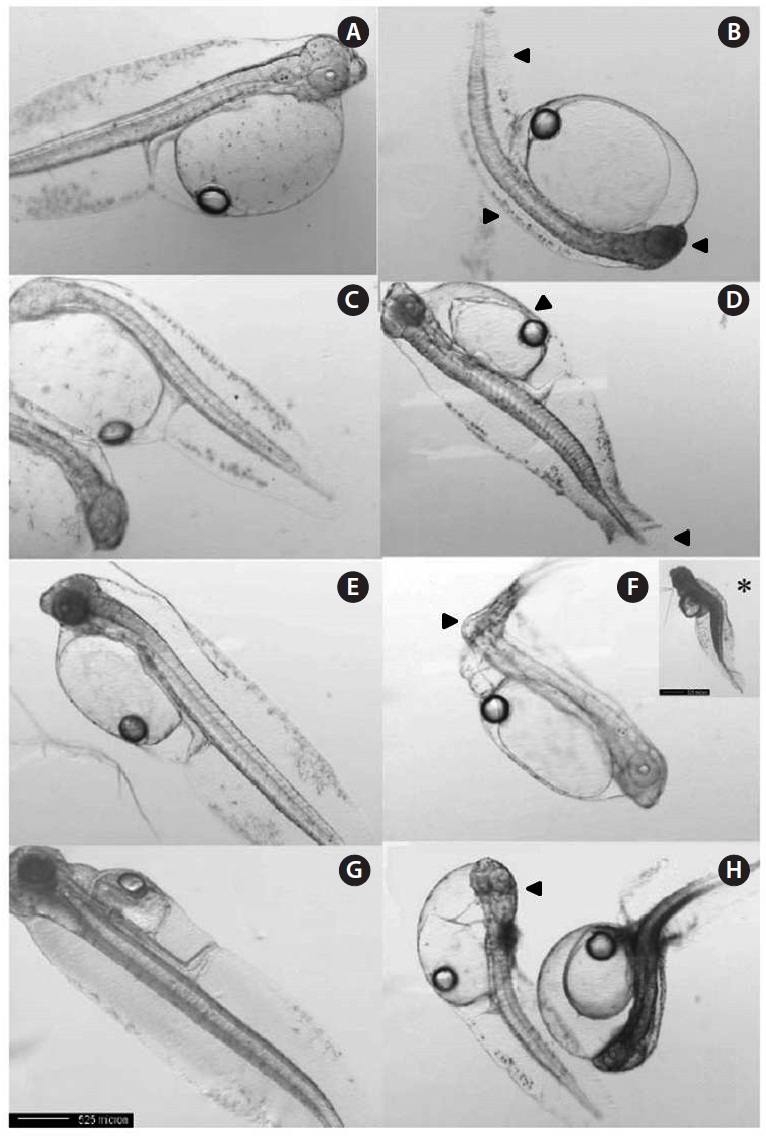
For larvae exposed to 1 μg/L, approximately 21% exhibited lateral curvature or spinal cord deformation (Fig. 7B shows larvae after 12-h exposure). Many of the surviving larvae displayed abnormal tail flexure and this became more pronounced with increased exposure (Fig. 7F and H show larvae after 48-h and 72-h exposure). All Aroclor 1254-treated groups exhibited ventral and caudal fin erosion, vertebral deformity, and abnormality of the yolk-sac (Fig. 7D, F, H). The incidence of gross lesions in larva exposed to Aroclor 1254 was similar to previous reports (Wilson and Tillitt, 1996), where larvae with craniofacial deformities, yolk-sac edema, and hemorrhaging was affected by 8.8 μg/kg total PCBs (Aroclor 1248, 1254, and 1260). Pericardial edema, yolk sac edema and hemorrhage in the liver rudiment occurred in the Japanese medaka after exposure to TCDD (2.5-12.3 pg/mL) and PCB 126 (0.05-0.8 μg/L). Aroclor 1254 produces similar toxicity effects to TCDD (Zable et al., 1995), polychlorinated dibenzodioxins and polychlorinated dibenzofurans in olive flounder larvae (Guiney et al, 1990). These effects, which are indistinguishable from blue-sac disease, start to appear about 12 h prior to hatching. They include yolk sac, pericardial and meningeal edema, craniofacial malformations, circulatory disruption, arrested growth and development, and ultimately death (Spitsbergen et al., 1991; Walker et al., 1994).
Black et al. (1988) reported that winter flounder larvae
We confirmed that olive flounder embryos were more tolerant than larvae of exposure to Aroclor 1254 and that malformations due to Aroclor 1254 were more common in the larvae than embryos. These results support the hypothesis of a protective role for the egg membrane against water-borne Aroclor 1254 (Noor et al., 1986).
This study highlights the potential susceptibility of the larval-stage compared to the embryo stage and stresses the importance of maintaining good water quality during production. It also demonstrates that the larval stage of the olive flounder would be a good indicator organism for Aroclor 1254 contamination in coastal waters, and that the response recorded may be typical for other contaminants (Fraysse et al., 2006). Finally, exposure to Arolcor 1254 concentrations as low as 1 μg/L disrupted early developmental stages of the olive flounder, with higher toxicity in the larvae than in the developing, unhatched embryos. To conclude, this study highlights the importance of testing different life stages and recording a range of endpoints when assessing the potential effects of contaminants on fish populations.