



A new interface for coupling CIEF and MS using a four-way cross has been developed in a single mechanical system. This new interface could be operated without the electric discontinuity and reinstallation of lines. Additionally, a bare fused sil-ica capillary was facilitated as a spray needle to produce electrospray and to guide catholyte or sheath liquid. Focusing for CIEF was completed in a hanging droplet at the end of spray needle. This capillary spray needle also provided stable spray, enhanced the ionization efficiency and increased sensitivity. Results with carbonic anhydrase I showed that focusing and spraying were well completed with the new interface and the new spray needle.
Conventional isoelectric focusing allows separating the amphoteric compounds such as peptides, proteins in slab gels according to their isoelectric point (p
Coupling CIEF with mass spectrometry (MS) by electrospray ionization was first demonstrated by Tang
In this study, a new interface for CIEF and MS was developed using a four-way cross to deliver the catholyte and the sheath liquid within a single mechanical system. Four-way cross system does not need to stop the electricity neither to move the spray tip when starting the operation of CIEF/MS. In addition, the bare fused silica capillary was employed as a spray needle to produce stable electrospray as well as to guide the sheath liquid. The result in loading carbonic anhydrase I (CA I) onto the CIEF capillary column showed this new four-way cross interface and the new spray needle operated well providing a peak in electropherogram. Coupling with CIEF and FTMS showed a charge and isotopic distribution in mass spectrum.
Ammonium hydroxide and acetic acid were purchased from Sigma (St. Louis, MO, USA). Pharmalyte (pH 3-10; GE Healthcare) was used as a carrier ampholyte to generate a pH gradient at 25 kV. Samples were desalted using Millipore Amicon Ultra-0.5 mL centrifugal filter and premixed with the carrier ampholyte (final concentration of 1%, v/v). Then samples were centrifuged at 12000 rpm for 10 min and subjected to sonication to remove gas bubbles.
The column length was 100 cm (70 cm effective length for CIEF-UV). Ammonium hydroxide (~1%, v/v, pH ~9.5) solution was used as the catholyte and acetic acid (~1%, v/v, pH ~2.6) solution was used as the anolyte. The entire column was filled with the sample which was prepared with 1% ampholyte. CIEF experiments were performed using PRINCE Crystal 650 CE system (Emmen, Netherlands) and UV detection (Lambda 1010 UV-Vis; Bischoff, Germany) was carried out at 280 nm. A constant voltage of 25 kV was applied during focusing (20 min) and hydrodynamic mobilization (~25 mbar). Mass spectra were acquired by 12 T Fourier Transform Mass Spectrometer (FTMS, Agilent, Lake Forest, CA, USA) in positive mode with 1.6 s scan rate. Mass range of FTMS was performed in m/z 600~2000. Sheath liquid solution consisted of 40% methanol, 58% water and 2% acetic acid (v/v/v) and delivered at a flow rate of 2 μL/min by a syringe pump. 3.5 kV was applied to the four-way cross for ESI spraying which was monitored by a CCTV camera to check the shape and stability of ESI.
>
Preparation of Coated Column
Hydroxypropyl cellulose (HPC) powder was dissolved in water at room temperature to a final concentration of 5% (w/w). The polymer solution was left overnight to eliminate bubbles. The capillary column was filled with the HPC solution by a syringe pump, and the excess solution was drained out by using N2 gas at 30 psi. The polymer layer coated on the capillary inner wall was immobilized by heating from 60 ℃ to 140 ℃ at 5 ℃/min and then keeping at 140 ℃ for 20 min in a gas chromatographic oven with N2 gas at 30 psi.
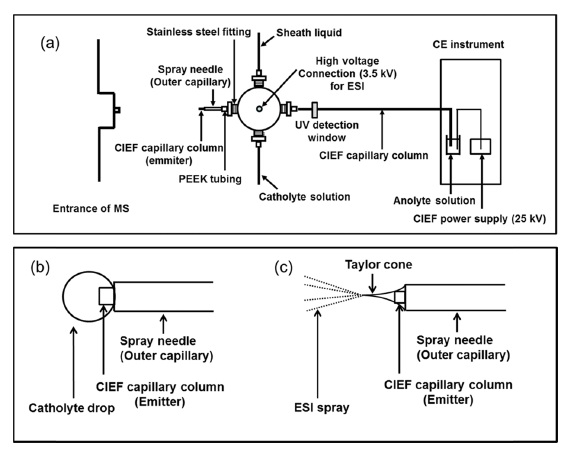
Figure 1(a) demonstrates a diagram of the four-way interface for CIEF-ESI-MS. The new interface employed the four-way cross for delivering the catholyte and the sheath liquid separately. A CIEF capillary column was inserted from one port of the four-way cross and passed through the spray needle (sheath liquid line) installed at the opposite port of the four-way cross. The capillary column was fixed to protrude
The electric circuit for focusing was completed by applying high positive voltage (25 kV) at the anode side and grounding the cathode side (the body of four-way cross which is connected to the hanging drop by sheath liquid inside spray needle). In this experiment, a catholyte ‘hanging drop’ forms and drops away for every 6 minutes when the catholyte flow kept at 2 μL/min. Once focusing was completed, the mobilization step started with starting sheath liquid flow and stopping catholyte flow. In this way, liquid co-axially flowing through the spray needle was switched from catholyte to sheath liquid without manual exchanging of lines and electrical discontinuity. Then, 3.5 kV applied to the cross for producing ESI spray (Figure 1 (c)) with maintaining 25 kV at the anode side for keeping the
In the early attempt for interfacing CIEF-MS, a commercial electrospray adapter kit or a modified metal tubing spray needle was employed to apply the high voltage for electrospraying.347 However, these metal spray needles
had limitation to provide a stable spray with high voltage causing electric arching between the spray needle and the entrance for mass spectrometry in CIEF-MS system. In this study, a bare fused silica capillary was used as a spray needle (outer capillary). The inner dimension of the capillary spray needle (I.D. 530 μm) was larger than the outer dimension of CIEF capillary column (O.D. 360 μm), therefore the CIEF capillary column pass through and protrude beyond it at the ESI side. This double capillary needle system provided stable spray, enhanced the ionization efficiency and increased sensitivity.
Figure 2(a) shows a typical electropherogram of carbonic anhydrase I (CA I:
In summary, the new interface for online CIEF-ESI-MS was developed using the four-way cross. The fused silica capillary outer tubing facilitated the focusing and stable electrospray transferring catholyte or sheath liquid. The catholyte hanging-drop at the end of capillary column leaded the complete focusing shown on the reconstructed electropherogram of CA I. This single mechanical system with four-way cross could carry out CIEF-ESI-MS without washing the line, discontinuity of electric circuit and reinstallation of ESI tip. Coupling FT/MS with CIEF shows the isotopic mass distribution as well as the charge distribution of CA I in the mass spectrum. With further optimization of the operational parameters of the new interface, this online CIEF-ESI-MS can be performed for the complex protein samples by achieving protein separation according to their
![A typical electropherogram of CA I (pI 6.6, 0.1 mg/mL) acquired by CIEF-UV (a). A reconstructed electropherogram of CA I was acquired by four-way interfacing online CIEF-ESI-FTMS (b). The difference of migration time for CIEF-ESI-FTMS from that for CIEF-UV was due to the difference of effective length of analytical capillary column. The MS scan was accomplished over the m/z range 600 - 2500 with scan rate 1.6 s. The MS scan spectrum at retention time 35.15 min (c) showing the charge distribution of CA I from +36 to +26. The enlarged MS spectrum showed the isotopic distribution of charge state of [M+29H]29+ (m/z 994) indicating the resolution of FTMS.](http://oak.go.kr/repository/journal/12965/E1MPSV_2013_v4n4_83_f002.jpg)