Many aquatic ecosystems are faced with spatially or temporally alarmingly high levels of xenobiotic chemicals (Brack et al., 2002; Diez et al., 2002). Tributyltin (TBT) is a typical synthetic pollutant, which was selected for investigation in this study. TBT is an organotin compound used primarily as a biocide in antifouling paints for ships, boats, and fishing nets. The use of TBT as an antifouling agent in paint has been banned for small boats and fishing nets in most countries as it has been shown to be toxic to aquatic life and is an endocrine disrupting chemical that generates severe reproductive effects in aquatic animals (Horiguchi et al., 1994). Reproductive impairment in gastropods has been reported at low TBT concentrations (Gibbs et al., 1988). TBT has been shown to induce imposex and intersex in brosobranchs (Oehlmann et al., 1998), and impaired reproductive function in fish has also been described (Mc Allister and Kime, 2003).
Some studies have reported toxic effects of TBT such as morphological and functional alterations of teleost gills in aquatic media (Byrne et al., 1989; Schwaiger et al., 1992; Tsuda et al., 1992; Wang and Huang, 1998). TBT has also been shown to markedly inhibit fish hepatic CYP and in particular the isozyme cytochrome P4501A1 (CYP1A), both in vivo and vitro (Fent and Bucheli, 1994; Morcillo and Porte, 1997). The CYP1A isozyme plays a key role in the biotransformation of xenobiotic compounds, leading to either their detoxification or bioactivation, while other CYP forms are important in the metabolism of hormones, fatty acids, and other critical endogenous molecules (Stegeman and Hahn, 1994). Inhibition of the CYP1A-catalyzed 7-ethoxyresorufin O-deethylase (EROD) and BaP hydroxylase (BPH) reactions by TBT could also interfere with their use as biomarkers of organic pollution in environmental monitoring (Goksoyr, 1995; Livingstone and Goldfarb, 1998; Whyte et al., 2000).
The rock bream is an economically important fish species in Korea that is commonly cultured for food in marine-based cages. Despite the importance of the rock bream in Korea, relatively little information is available regarding the effects of TBT on the species. Therefore, this study investigated the responses of the xenobiotic biotransformation system in the hepatic tissues of rock bream following chronic waterborne TBT exposure. The study evaluated the effects of TBT on the cytochrome P450 monooxygenase system in rock bream, and the findings suggest that the species can be used as an indicator of the TBT toxicity level in water systems.
Rock bream individuals were obtained from a fish farm in Gyeongsangnam-do, Korea. Fish were held for 3 weeks to acclimatize and to evaluate the overall fish health under laboratory conditions (20.2 ± 0.34℃) prior to exposure. During the acclimation period, fish were fed with a commercial diet twice daily and maintained on a 12:12 h light/dark cycle at all times. After acclimatization, fish (body length, 12.8 ± 0.32 cm; body weight, 33.7 ± 0.65 g) were selected for the experiments.
The exposure took place in 30-L glass tanks (500 × 280 × 310 mm) containing 20 fish in each treatment group. Each tank received a water flow of 7 L min–1 with continuous aeration. Tributyltin chloride (96% purity) was purchased from Sigma-Aldrich Chemie GmbH (Steinheim, Germany). A stock solution of TBT was prepared by diluting 0.02 g TBT in 1 mL acetone before use. Acetone is known to be nontoxic in terms of histopathological effects (Fent and Meier, 1992). TBT was added to the experimental tanks at final concentrations of 0, 1, 2, 4, and 8 μg/L for 4 weeks as a chronic exposure. All experiments were conducted using a static system. TBT solutions in each experimental tank were renewed once daily.
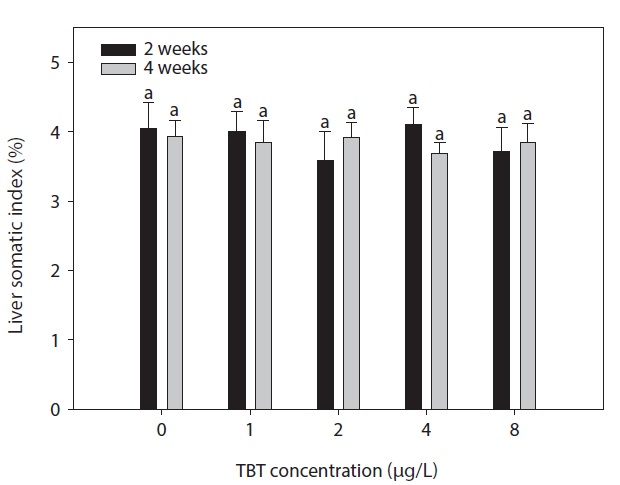
The hepatosomatic index (HIS) was determined as the ratio of liver weight to body weight.
HSI (%) = liver wet weight/weight*100
Hepatic cytochrome P450 content was determined from the carbon monoxide–oxide difference spectra of dithionite-reduced microsomes using an extinction coefficient of 91 mM–1 cm–1 for A450–A490 (Omura and Sato, 1964).
Hepatic EROD activity was determined using a modification of the multiwell plate method (Kennedy and Jones, 1994). The following reagent concentrations were used in the EROD reaction mixture: 7-ethoxyresorufin, 1.7 µM (Sigma, St. Louis, MO); NADPH, 0.5 mM (Boehringer Mannheim, Mannheim, Germany); MgSO4, 17 mM; and HEPES, 0.1 M, pH 7.8 M (Sigma). EROD activity was determined in 48-well culture plates (Costar, Cambridge, MA) using a plate reading flourometer (BF10001, Packard Bioscience Co., Meriden, CT). Excitation and emission filters were set at 530 nm and 590 nm, respectively. The activity was determined at 25℃ using 25 μL postmitochondrial supernatant in the reaction mixture. The penthoxyresorufin
Statistical analysis of the results was performed using the SPSS/PC+ statistical package (SPSS Inc., Chicago, IL). ANOVAs and Duncan’s test for multiple comparisons were used to test for significant differences between the control and treatment (Duncan, 1955). The significance level was set at
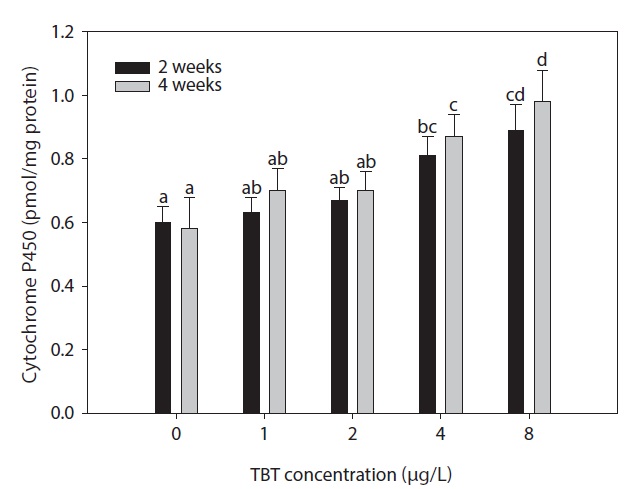
The effect of waterborne TBT exposure for 4 weeks on the HSI of rock bream is shown in Fig. 1. No differences were observed in the HSI of treated groups compared to the control. Fig. 2. shows the effect of waterborne TBT on hepatic CYP450 content in rock bream. Hepatic CYP450 content in the 0, 1, and 2 μg/L-treated groups were stable during the experimental period. However, the hepatic CYP450 content significantly increased in the 4 μg/L-treated group after 2 weeks exposure to TBT (
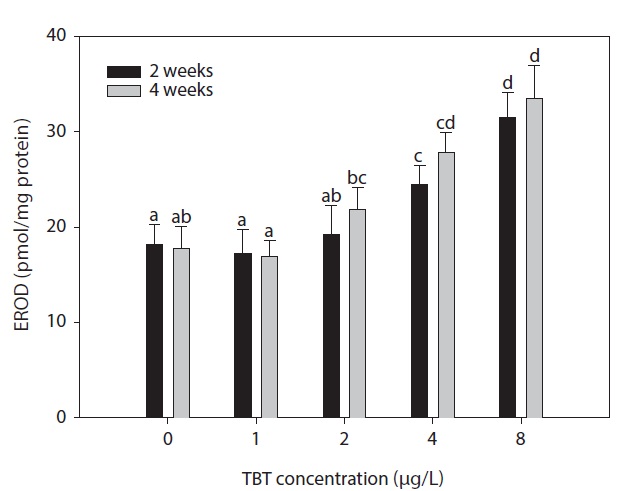
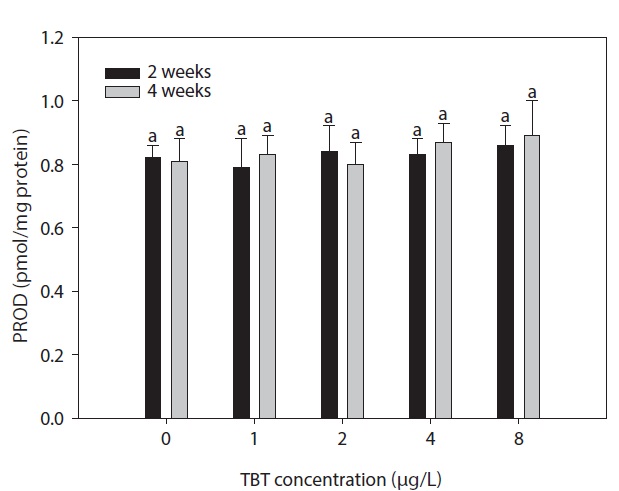
4 weeks was observed in the groups treated with TBT concentrations of more than 2 μg/L. Fig. 4. shows the effects of TBT on PROD activity in the hepatic microsomes of rock bream. No significant changes were observed in all the treated groups compared to the control group (
The results clearly indicate that exposure of rock bream to TBT induced hepatic cytochrome P450 and de-alkylase activity. However, no significant change occurred in the HSI. A similar result for was observed previously in common carp supplemented with anthraquinone (Xie et al., 2008).
The duration and intensity of action of xenobiotics within a biological system are determined by the rate of their biotransformation to pharmacologically active or inactive metabolites. Cytochrome P450, a heme protein, is a heterogeneous system of microsomal enzymes responsible for the oxidative biotransformation of many chemicals to polar metabolites, thereby facilitating the pharmacological inactivation of these chemicals and their elimination from the body (Lu and West, 1980; Wrighton and Stevens, 1992). Cytochrome P450 occurs in multiple forms and the composition of these isozymes, as well as their relative concentrations in tissues, are influenced by treatment with different chemicals (Lu and West, 1980). Several studies have observed that TBT suppresses cytochrome P450 1A in fish. The influence of TBT on hepatic cytochrome P450 levels in aquatic organisms has been studied in practical situations (Rice and Roszell, 1998).
In this study, a significant increase in hepatic cytochrome P450 level was observed in groups exposed to TBT. The hepatic cytochrome P450 content of the 4 μg/L-exposure group was significantly different from the control for an exposure duration of either 2 or 4 weeks. At the end of the experiment, the cytochrome P450 content of the 8 μg/L-exposure group was higher than in the control group. A pronounced increase in hepatic cytochrome P450 induction occurred in the presence of TBT, which confirms that cytochrome P450 induction in the rock bream can be a reliable indicator of contamination of the aquatic environment by compounds known to induce CYP1A, including many xenobiotics (Colloer et al., 1995; Yolanda et al., 2004).
Hepatic EROD activity in rock bream is increasingly used to indicate the presence or effects of certain organic contaminants. This is because laboratory studies have established a strong causal link between exposure of the fish to contaminants and the expression of cytochrome P4501Al and EROD activity (Haasch et al., 1989; Skaare et al., 1991). Although monitoring of cytochrome P4501Al and EROD activity has been widely used as biomarkers of aquatic pollution, the effect of TBT on these parameters has not yet been established. Our results have shown that following waterborne exposure of TBT (8 μg/L), significant induction of hepatic EROD occurred after 4 weeks.
In this study, no significant difference between TBT-treated and control groups in terms of hepatic PROD activity was observed. PROD activity is a catalytic probe for determining the induction response of CYP2B class isozymes in mammals. In the P450 system of fish, the phenobarbital (PB)-type inductive response appears to be completely absent (Goksøyr and Förlin, 1992). CYP2B genes have been established to be present and expressed in fish, but also that they are nonresponsive to PB-type compounds (Stegeman et al., 1990). In a study by Brown (1992), a large series of gas chromatograms of polychlorinated biphenyl (PCB) residues in 32 species of teleost fish was investigated. CYP2B-like alteration patterns were observed in only four species. In this study, insignificant PROD induction was detected in the rock bream exposed to TBT. Therefore, TBT does not induce hepatic CYP2B class isozyme. In conclusion, waterborne TBT significantly affected the cytochrome P450 content and EROD activity. The level of the decrease induced by chronic exposure to TBT can be used as a biomarker in coastal and estuarine risk assessments.