We investigated the antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of red snow crab Chionoecetes japonicas shell (RSCS) hydrolysate by enzymatic hydrolysis and its molecular weight cut-off fractions. The RSCS hydrolysate was fractionated through two ultrafiltration membranes of 3 and 10 kDa cut-offs. Three fractions (<3 kDa, 3-10 kDa, and>10 kDa) were evaluated for total amino acid composition, antioxidant activities using 2′-azino-bis[3-ethylbenzthiazoline-6-sulfonic acid] (ABTS+) radical scavenging and superoxide dismutase (SOD)-like activities and reducing power assays, and ACE inhibitory activity using Hou’s method. Although all fractions showed activity, the<3 kDa fraction of RSCS hydrolysate exhibited the greatest ABTS+ radical scavenging, SOD-like and ACE inhibitory activities. However, these fractions exhibited low reducing power. These results suggest that the low-molecular-weight enzymatic hydrolysate of RSCS could be used as a functional ingredient to control oxidative stress and ACE activity.
Blood pressure is regulated by various controlling factors including angiotensin I converting enzyme (ACE, EC 3.4.15.1). This enzyme is a bivalent dipeptidyl carboxyl metallopeptidase, which converts angiotensin I, an inactive decapeptide produced from angiotensinogen, into angiotensin II, a vasoconstrictive octapepide, and inactivates the vasodilator bradykinin, thus interacting simultaneously with the renin-angiotensin and kallinkrein-kinin systems (Mäkinen et al., 2012). ACE inhibitors are one of the most effective methods for suppressing increases in blood pressure and have a long history of use, with interest in them continuing to increase (Pfeffer and Frohlich, 2006).
Oxidative stress occurs due to an imbalance between oxidizing species and natural antioxidants or antioxidant enzymes, and has been implicated in the occurrence of hypertension and a number of pathological conditions, including neurodegenerative diseases, diabetes, cancer, and aging (Beckman and Ames, 1998; Giasson et al., 2002). Reactive oxygen species (ROS), such as the superoxide anion radical (O2−), hydrogen peroxide, and hydroxyl radicals (·OH) are generated from the sequential reduction of oxygen during respiration in aerobic organisms. ROS are highly unstable, and react rapidly with other substances, including DNA, membrane lipids and proteins, and may cause cellular damage leading to apoptosis, aging, and inflammation (Je et al., 2009). Although the body has its own defense system against ROS, it cannot prevent oxidative damage completely. Thus, food supplements containing antioxidants can be used to reduce oxidative damage.
Red snow crab
The aim of this study was to determine the effect on antioxidant and ACE activities of fractions of red snow crab shell (RSCS) hydrolysate of various molecular weights.
RSCS was purchased from a red snow crab processing plant (Sokcho, Korea) in July 2012. The lyophilized shell powder was stored in a freezer at -20℃ until use. 2,2-azino-bis (3-ethylbenzthiaxoline)-6-sulfonic acid (ABTS+), o-phthaldialdehyde, pyrogallol, potassium persulfate, trolox, potassium ferricyanide, trichloroacetic acid, ferric chloride, angiotensin I converting enzyme, N-[3-(2-furyl) acryloyl]-Phe-Gly-Gly (FAPGG), captopril, and L-ascorbic acid were purchased from Sigma Chemical Co. (St. Louis, MO, USA) and Alcalase 2.4 L was purchased from Novo Co. (Novozyme Laboratories, Copenhagen, Denmark). All other reagents were of the highest grade available.
>
Enzymatic hydrolysis and fractionation
RSCS was used for enzymatic hydrolysis. Its crude protein content was 27.37% as determined by the Kjeldahl method. Alcalase 2.4 L was used to prepare RSCS protein hydrolysate under optimal conditions (50℃, pH 7.0). A sample (100 g) and enzyme (0.27 g) were mixed and hydrolyzed at 50℃ for 25 h. After 25 h, the mixture was heated at 100℃ to inactivate the enzyme. Unhydrolyzed proteins were removed by passing the hydrolysate through filter paper, and the supernatant was lyophilized (13.70 g) and stored at -20℃ until use.
The lyophilized hydrolysate (12.70 g) was dissolved in deionized water at 50 mg/mL. The solution was filtered by two ultrafiltration membranes (Amicon Ultra-4 filter devices; Millipore, Billerica, MA, USA) with 3 and 10 kDa molecular weight cut-offs (MWCO), yielding three fractions corresponding to molecular weights<3 kDa (5.80 g), 3-10 kDa (4.72 g), and>10 kDa (2.18 g). The soluble fractions were prepared by centrifuging at 3,000 rpm for 20 min and were passed through the membranes sequentially, beginning with the largest MWCO membrane cartridge (10 kDa). The retentate and permeate were collected separately, and the retentate was recirculated into the feed until the maximum permeate yield was reached. Permeate from the 10 kDa membrane was then filtered through the 3 kDa membrane with recirculation until the maximum permeate yield was reached.
The DH was calculated by determining free amino groups with
DH = h/htot × 100
, where htot is the total number of peptide bonds per protein equivalent, and h is the number of hydrolyzed bonds. The htot factor is dependent on the amino acid composition of the raw material.
The total amino acid composition was determined using an amino acid analyzer (S43000; Sykam, Eresing, Germany). Samples were hydrolyzed in 6 N hydrochloric acid in vacuum-sealed tubes at 110℃ for 24 h.
>
ABTS+ radical scavenging activity
ABTS+ radical scavenging activity was determined by modifying the method of Arnao et al. (2001). The stock solutions were 7.4 mM ABTS+ and 2.6 mM potassium persulfate. The working solution was prepared by mixing the two stock solutions in equal quantities. The mixture was allowed to react for 12 h at room temperature in the dark, followed by dilution by mixing 1 mL ABTS+ solution with 50 mL MeOH to an absorbance at 734 nm of 1.10 ± 0.02, as determined using a spectrophotometer (BioMate 5; Thermo Electron, Waltham, MA, USA). Fresh ABTS+ solution was prepared for each assay. A sample (150 μL) was mixed with 2.85 mL ABTS+ solution and the mixture was left in the dark for 2 h. The absorbance at 734 nm was then measured using a spectrophotometer. Trolox was used as a positive control. ABTS+ radical scavenging activity was calculated using the following equation:
Scavenging activity (%) = [1 – {(A – B) / C}] × 100
where, A = Absorbance of sample/Standard with reagent, B = Absorbance of sample/Standard without reagent, and C = Absorbance of the control.
>
Superoxide dismutase (SOD)-like activity
The SOD-like activity of RSCS hydrolysate was determined using the method described by Marklund and Marklund (1974) with a slight modification. Briefly, 200 μL of sample solutions were mixed with 200 μL of pyrogallol solution (7.2 mM in water) and 3 mL of 50 mM Tris-HCl buffer, at pH 8.5, containing 10 mM EDTA. After 10 min, 1 mL of 1 N HCl was added to the above mixture to stop the reaction. L-ascorbic acid was used as a positive control. The absorbance was measured at 420 nm. SOD-like activity was calculated using the following equation:
Scavenging activity (%) = [1 – {(A – B) / C}] × 100
where, A = Absorbance of sample/Standard with reagent, B = Absorbance of sample/Standard without reagent, and C = Absorbance of the control.
Reducing power was evaluated by the method of Oyaizu (1986). Various sample concentrations (2.5 mL) were mixed with 2.5 mL of 200 mM sodium phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. After incubation at 50℃ for 20 min, 2.5 mL of 10% trichloroacetic acid (w/v) were added. The mixture was then centrifuged at 2,000 g for 10 min, and 5 mL of the upper layer were mixed with deionized water and 1 mL of 0.1% ferric chloride. The sample concentration resulting in 0.5 of absorbance (EC50) was calculated from the graph of absorbance at 700 nm, which was measured using a spectrophotometer (BioMate 5). L-ascorbic acid was used as a positive control.
The inhibition of ACE activity was conducted according to the method of Hou et al. (2003), and modified to use FAPGG as the substrate. In brief, FAPGG (0.5 mM) and various concentrations of the samples were completely dissolved in 50 mM Tris-HCl buffer (pH 7.5). 20 μL of ACE (1 U/mL dissolved in 50 mM Tris-HCl buffer) were then mixed with 200 μL of samples of various concentrations as experimental samples, or mixed with 50 mM Tris-HCl buffer as a negative control. Following the addition of 1 mL of 0.5 mM FAPGG to the reaction mixture, the optical density was determined after 0 and 30 min at a wavelength of 345 nm. The ACE inhibitory activities were expressed as the 50% inhibition concentration (IC50). The values of percentage inhibition were then calculated using the equation:
Inhibitory activity (%) = {1 – (ODsample/0min – ODsample/30min ) / (ODcontrol/0min – ODcontrol/30min)} × 100.
The antihypertensive agent captopril was used as a positive control.
The data were analyzed using analysis of variance through the general linear model procedure (SAS Institute, Cary, NC, USA). Duncan’s multiple-range test was applied to determine the significance of differences between means (
>
Enzymatic hydrolysis and fractionation
The bioactive protein hydrolysates depend on the protein substrate used, the specificity of the enzyme used, the conditions used during hydrolysis and the DH value (Nielsen, 1999; Kristinsson and Rasco, 2000). Because enzymes have specific cleavage positions on polypeptide chains, Alcalase has been used to produce bioactive peptides from processing by-products (Guerard et al., 2007).
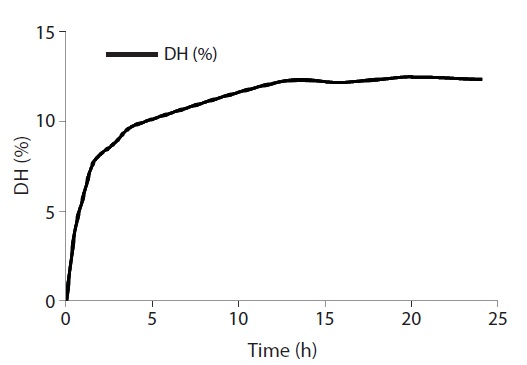
The extent of protein degradation by proteolytic enzymes was estimated by assessing the DH. As shown in Fig. 1, the rate of enzymatic hydrolysis of RSCS increased rapidly for the first 2 h and then entered a steady-state phase after 12 h with a DH of 12.5%. Alcalase hydrolysate was separated using two filters of MWCO 3 and 10 kDa to yield three fractions (<3 kDa, 3-10 kDa, and>10 kDa).
>
Total amino acid composition
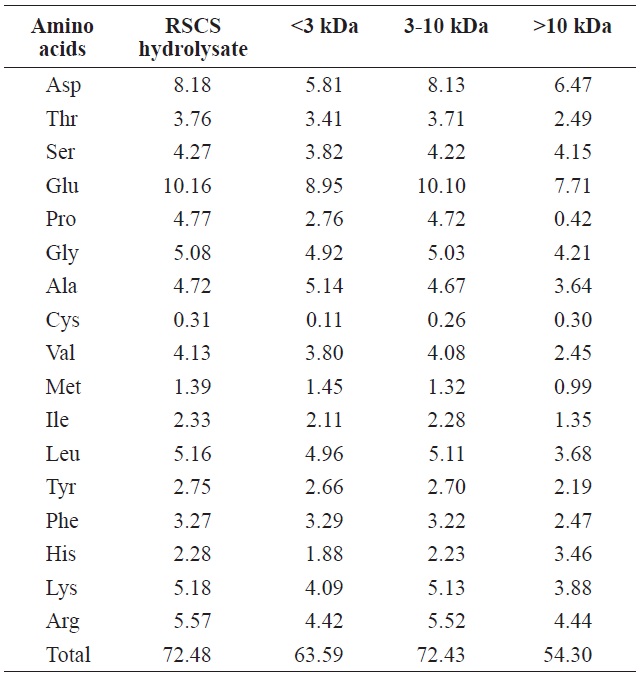
The total amino acid compositions of RSCS hydrolysate and its fractions are presented in Table 1. The total amino acid content of RSCS hydrolysate was 72.48 g/100 g, which was the highest of any fraction (Table 1). However, the total amino acid compositions of RSCS hydrolysate and its three fractions were similar. All hydrolysate fractions were rich in Glu, Asp, Gly, and Ala, but contained low levels of Met and Cys.
The carboxyl ends of Glu, Met, Leu, Tyr, and Lys in the peptide linkages were found to be preferentially cleaved by Alcalase (Adamson and Reynolds, 1996). Of these, Glu was the major amino acid in RSCS hydrolysate, which made it a good substrate for Alcalase (Yang et al., 2011).
Oxidative stress is a common risk factor in a number of chronic diseases, such as hypertension, diabetes, arthritis, neurodegenerative disease, and cardiovascular complications (Bernardini et al., 2011; Je et al., 2005). Excessive production of ROS causes adverse effects in cells, such as oxidation of bio-molecules. Therefore, ROS production must be regulated by antioxidants and antioxidant systems (Himaya et al., 2012).
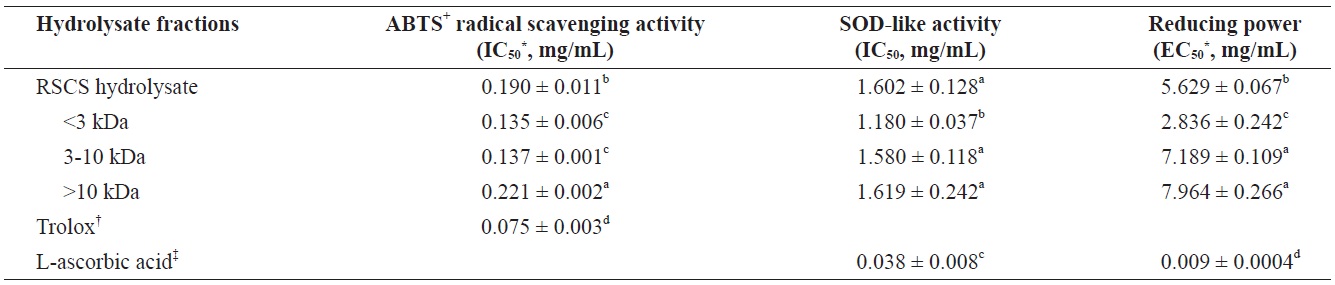
The antioxidant activities of RSCS hydrolysate and its three MWCO fractions were evaluated to determine their ABTS+ radical scavenging activity, SOD-like activity, and reducing power (Table 2). The<3 kDa fraction exhibited the greatest antioxidant activity.
The scavenging activities of RSCS hydrolysate and its three fractions toward ABTS+ radicals were as follows:<3 kDa fr. (IC50 = 0.135 ± 0.006 mg/mL)>3-10 kDa fr. (IC50 = 0.137 ± 0.001 mg/mL)>RSCS hydrolysate (IC50 = 0.190 ± 0.011 mg/mL)>>10 kDa fr. (IC50 = 0.221 ± 0.002 mg/mL) (Table 2).
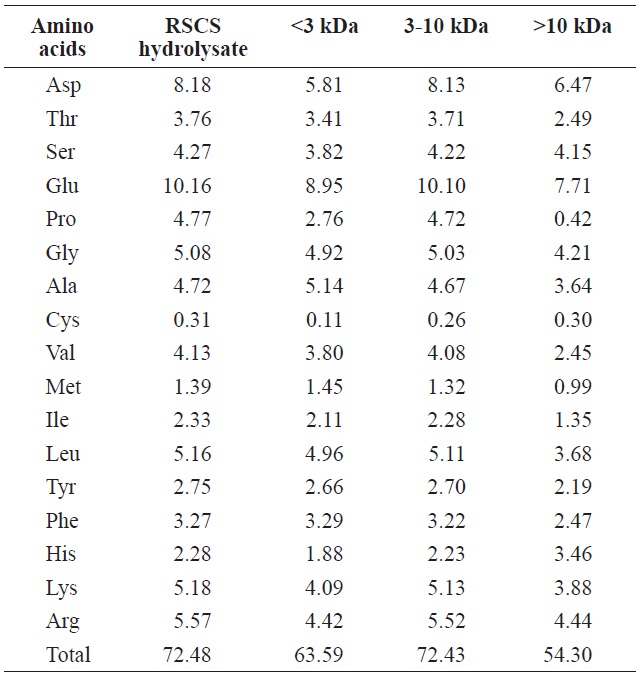
Total amino acid composition of RSCS hydrolysate and its molecular weight cut-off fractions (g/100 g)
[Table 2.] Antioxidant activities of RSCS hydrolysate and its molecular weight cut-off fractions
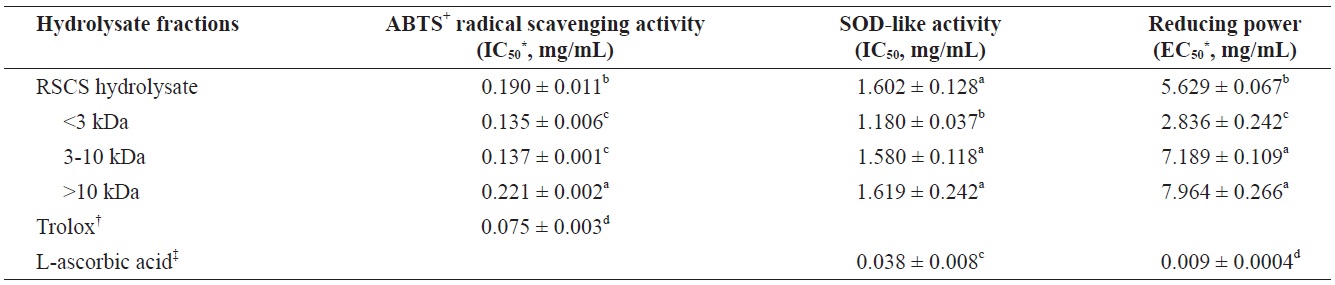
Antioxidant activities of RSCS hydrolysate and its molecular weight cut-off fractions
Thus the<3 kDa fraction was the most potent scavenger of ABTS+ radicals. The 3-10 kDa and>10 kDa fractions, and RSCS hydrolysate, also exhibited noticeable scavenging activity.
SOD is an antioxidative enzyme that catalyzes the breakdown of the superoxide anion into oxygen and hydrogen peroxide. This is a widely used assay to predict the SOD-like antioxidant activity, and is based inhibition of the auto-oxidation of pyrogallol. RSCS hydrolysate and the three MWCO fractions exhibited SOD-like activity with IC50 values of 1.602 ± 0.128, 1.180 ± 0.037, 1.580 ± 0.118, and 1.619 ± 0.242 mg/mL, respectively (Table 2). Of these, the<3 kDa fraction showed the greatest SOD-like activity. However, all fractions exhibited lower SOD-like activity than the positive control, L-ascorbic acid.
Reducing power is the ability to reduce ferric (Fe3+) to ferrous (Fe2+), which can be a direct correlation between antioxidant activity and the reducing power of bioactive constituents (Dorman et al., 2003).
The RSCS hydrolysate and its three fractions had electron donation capacity (Table 2). The<3 kDa fr. (IC50 = 2.836 ± 0.242 mg/mL) exhibited the greatest reducing power. However, all fractions showed lower reducing power than the positive control, L-ascorbic acid.
The differences in antioxidative activity among hydrolysates of different molecular weights might be due to their diverse amino acid compositions (Wu et al., 2003). Aromatic amino acids;
The ACE activation causes a rise of blood pressure by increasing vascular resistance and fluid volume, while ACE inhibitors exert an antihypertensive effect (Zhao and Li, 2009). Synthetic ACE inhibitors, including captopril, enalapril, alacepril,
[Table 3.] ACE inhibitory activity of RSCS hydrolysate and its molecular weight cut-off fractions
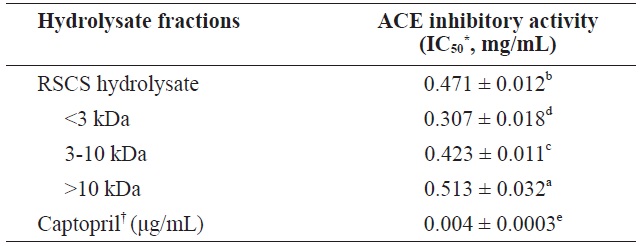
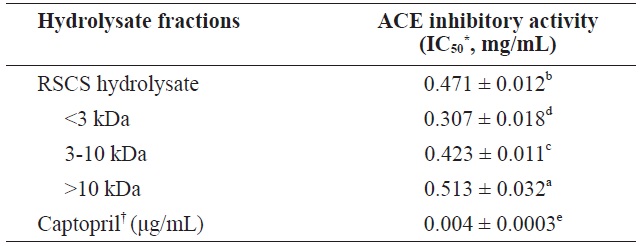
ACE inhibitory activity of RSCS hydrolysate and its molecular weight cut-off fractions
fosinopril, and lisinopril, have been used for the treatment of hypertension and heart failure (Brown and Vaughan, 1998). However, these synthetic antihypertensive agents cause side effects such as coughing, taste alterations, skin rashes, and angioneurotic edema (Alderman, 1996; Cicoira et al., 2001; Vyssoulis et al., 2001). Therefore, much interest has focused on the use of safe ACE inhibitors derived from natural products to reduce side effects.
The ACE inhibitory activity of RSCS hydrolysate and its MWCO fractions is shown in Table 3. The<3 kDa fraction exhibited the highest ACE inhibitory activity (IC50 = 0.307 ± 0.018 mg/mL), followed by the 3-10 kDa fraction (IC50 = 0.423 ± 0.011 mg/mL), RSCS hydrolysate (IC50 = 0.471 ± 0.012 mg/mL), and the>10 kDa fraction (IC50 = 0.513 ± 0.032 mg/mL). These results indicate that the order of the inhibition of ACE activity is similar to that for antioxidant activities.
In recent years, inhibition of ACE and antioxidant activity has been reported for bioactive peptides isolated from processing by-products such as Alaska pollock skin gelatin (Kim et al., 2001), yellow fin sole frame (Jung et al., 2006), big eye tuna dark muscle (Qian et al., 2007), tuna frame protein (Lee et al., 2010), Pacific cod skin gelatin (Himaya et al., 2012), hoki frame protein (Kim et al., 2007), Nile tilapia skin gelatin (Ngo et al., 2010), and tuna backbone protein (Je et al., 2005). Most of these active peptides were of low molecular weight, in the range 1-6 kDa.
In conclusion, RSCS enzymatic hydrolysate and its MWCO fractions showed antioxidant activity and inhibited ACE activity. More detailed investigations are necessary to isolate and identify the active constituents of the hydrolysate and to determine the mechanism of action.