The human wound healing response consists of several complicated phases [1], including coagulation, inflammation, granulation,contraction, and regeneration of tissues. Prior to a wound entering into recuperation, each of these phases is affected by both internal and external factors such as medications, vitamins,radioactive treatments, and rate of collagen synthesis [2]. The occurrence of any wound should be carefully treated and managed,as they can cause various medical complications including inflammation. To this end, many methods of healing wounds exist,among which includes light therapy, which utilizes beams of light to assist the human body in treating and sterilizing wounds as well as regenerating cells.
In general, light therapy, which is also known phototherapy, is defined as a medical treatment that utilizes light sources, especially non-visible light such as ultraviolet and/or infrared light for the treatment of diseases and injuries. Ultraviolet therapy uses chemically active UV rays; however, rays in the ultraviolet spectrum (UVB, UVC’320 nm) are absorbed by proteins and DNA of dermal tissues, which can result in ionization and poses a risk of fever and decreased biosynthesis, especially when the skin is exposed to UVA and UVB radiation, which can result in skin aging and damage [7,8]. Likewise, infrared phototherapy utilizes infrared light rays to apply thermal conductivity.
Lasers with specific wavelengths such as He-Ne and GaAlAs lasers induce proliferation of fibroblasts depending on the size of the wound area or specific wavelength utilized [9] and are effective for pain relief [10], anti-inflammation [11,12] and wound healing [13-17]. Vinck et al. [18] reported that both LED irradiation and low level laser treatments induce increased cellular proliferation in specific cells, although cells treated with LED irradiation have a higher rate of proliferation than cells treated with low level lasers. Many studies on the effects of low power lasers have been performed in both cell culture and animal models, although the results of such studies are widely varied, making it difficult to verify the true effects of such treatments. In general however, the trends of such laser and LED studies indicate that while laser treatments are expensive and generate heat problems, LED treatments are economically effective and safe.
[Table. 1.] Conditions for the 525 nm green LED irradiation group.

Conditions for the 525 nm green LED irradiation group.
In the present study, we evaluated the wound healing effects of 525 nm green LED irradiation on skin wounds of Male Sprague Dawley Rats (ILAR CODE: NTacSam:SD).
The experiments in this study utilized 8 week old male adult 250 ~ 300 g Sprague Dawley Rats(ILAR CODE: NTacSam:SD). For animal care, we attempted to minimize stressful factors while checking for injury and disease and reduced environmental changes during the test period. The breeding chamber was automatically adjusted to 21 to 23℃ and the test chamber that was subjected to the 525 nm green LED irradiation was kept constant at 20℃. To generate even sized wounds, a forcep-style tool was made that was affixed to a 1cm diameter circular blade. Using this device, excision wounds were created by removing the epidermal and dermal layers. Wounds were made on both the right and left sides of animals.
To evaluate the effect of the 525 nm green LED irradiation on wound healing by splitting rats into either a non-irradiation group or 525 nm green LED irradiation group (n of each group=7); for the experimental treatment group we used an independently-developed 525 nm green LED irradiator. Animals used for experiments were allowed to rest for 24 hours after wounds had been excised, followed by either 525 nm green LED irradiation therapy or no-treatment over 9 days one hour per day. Table 1 describes the test conditions for the 525 nm green LED irradiation group.
Tissues were obtained for observation, fixed with 10% neutral formalin, sliced in 4-5 ㎛ thick specimens by paraffin embedding,and double stained with hematoxylin-eosin and treated with immunohistological staining against pan-cytokeratin and PCNA. Massons’ trichrome staining was carried out to evaluate tissue regeneration in defect areas.
For immunohistological examinations, we used mouse monoclonal antibodies for pan-Cytokeratin (C-11), Actin(C-2) and PCNA (PC10) manufactured by Santa Cruz Biotechnology Co. Ltd(Santa Cruz, CA, USA) at a diluting ratio of 1:200, respectively. For secondary immunostaining and visualization, we used the Labeled Streptavidin-Biotin (LSAB) Kit and Diaminobenzoic (DAB)acid kit manufactured by Dako Co. Ltd.(Glostrup, Denmark), respectively.Contrast staining was conducted by using Mayer’s hematoxylin while inclusion was visualized with Universal Mount developed by Research Genetics (Huntsville, AL, USA).
Paraffin embedded tissue, which was fixed with 10% buffered neutral formalin, was microtombed to a 4 ㎛ thickness and affixed to X-traTm slides (Surgipath, Richmond, U.S.A.).Paraffin removed with xylene which was followed by sequential treatment with 100%, 90%, 75%, and 50% ethanol for 2 minutes each, respectively, and staining using the LSAB method. Next, to recover antigenicity, slides were soaked in citrate buffer (10 mM,pH 6.0) heated by microwave oven for 15 minutes, then kept at room temperature for 20 minutes and rinsed with 50 mM Tris (TBS, pH 7.5). Slides were then treated with 0.3% hydrogen peroxide-methanol for 10 min in order to suppress endogenous peroxidase in the sliced tissue and then washed off with distilled water. Next, slides were allowed to respond to blocking antibody for 10 min, followed by primary antibody staining for 1 hour at room temperature. Following the reaction with the primary antibody,slides were washed with Tris buffer and allowed to react with biotin-containing secondary antibody for 10 min at room temperature, after which they were rinsed off with Tris buffer and soaked in streptavidin solution containing peroxidase for 10 min. Following this procedure, slides were again rinsed with Tris buffer, and antibody color staining and contrast staining were visualized with a DAB kit and Mayer’s hematoxylin and inclusion stain with universal mount, respectively. A positive contrast group was not separately prepared since neighboring normal tissues can serve as a positive control group. Likewise, a Tris buffer treated negative contrast group was used to compare primary antibody staining.
[Table. 2.] Defect size (Mean ’ SD).

Defect size (Mean ’ SD).
[Table. 3.] Collagen volume by groups.

Collagen volume by groups.
The defect area was measured using a computer measurement program based on a specimen that was double stained with hematoxylin-eosin. In cases where it was difficult to distinguish the marginal area of cellular regeneration from the defect area depending on cases, measurements were made with a slide that was immunohistologically stained against pan-cytokeratin.Measurement areas were photographed with a Magna Fire digital camera system (Optronics, Goleta, CA, USA) and the long dia meter of the defect area in the digital image was measured by using the iVisus Image Analysis System (Image & Microscope Technology, Daejon, Korea).
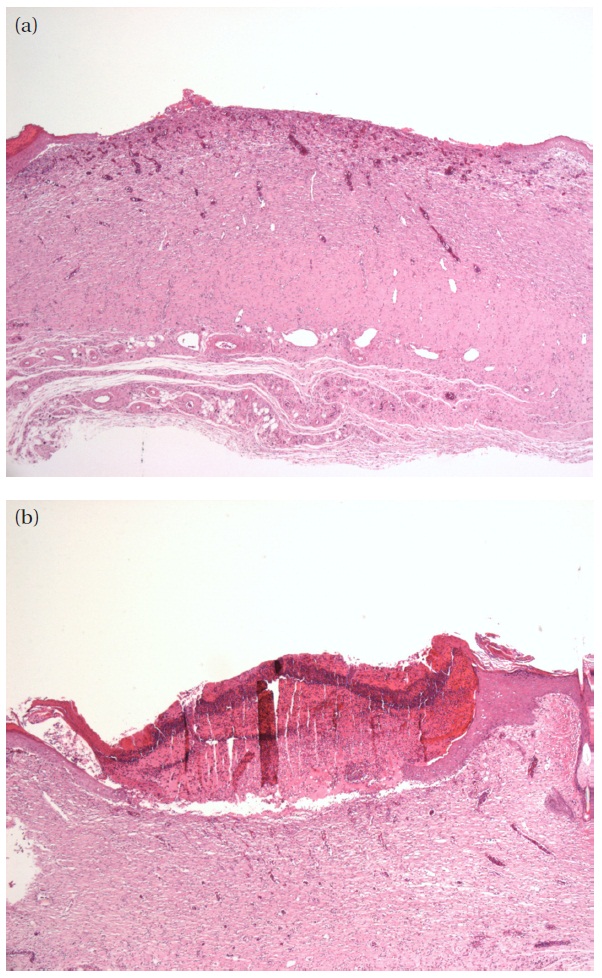
In order to examine the effect of the 525 nm green LED irradiation on wound healing, we first measured the respective sizes of defects in the group treated with 525 nm green LED irradiation and the non-irradiation group. Next, immunohistochemical staining was performed for cytokeratin in order to more precisely measure the defect size. Measurements of defect sizes (mean ± S.D) after treatment with 525 nm green LED irradiation or non-irradiation are shown in Table 2. A difference was observed between the 525 nm green LED irradiation group and the non-irradiation group. Specifically, the defect size in the 525 nm green LED irradiation group was decreased compared with the non-irradiation group (P<0.05). Figure 1 describes images of representative defect size in the non-irradiation group and the LED irradiation group.
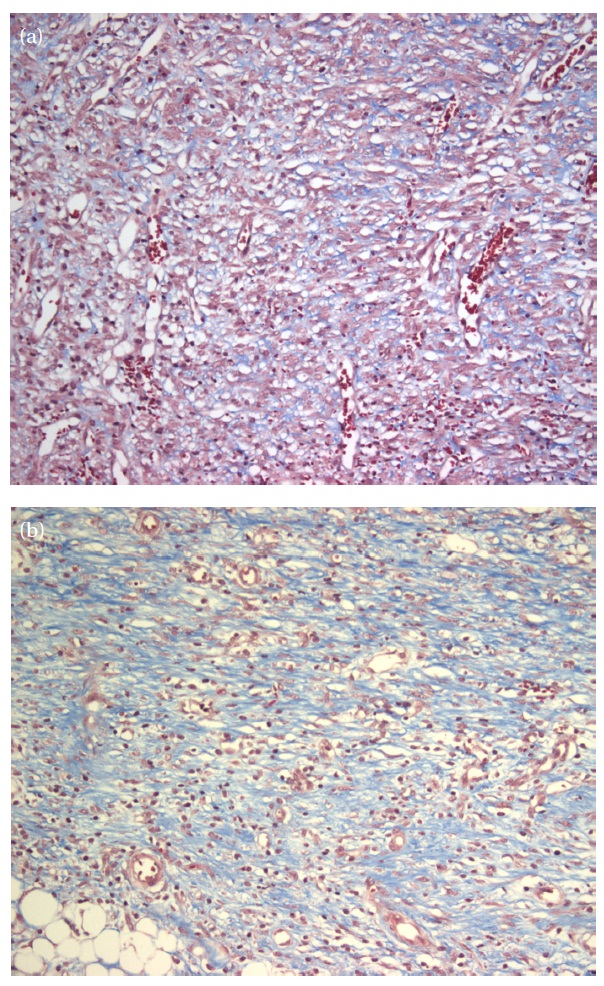
Masson’s trichrome staining was performed in order to compare collagen between the 525 nm green LED irradiation group and the non-irradiation group and to examine collagenosis and regeneration of cells in the defect area. Semi-quantitative scoring was performed depending on the quantity of collagen observed.Specifically, the results were scored 0 when no collagen was observed,1+ when the visible strands of collagen were minute, 2+when the small collagen bundles were scattered, 3+ when there were small or large bundles, and 4+ when juicy matrixes of collagen were partially formed. Table 3 shows collagen volume by groups. The results of our analysis showed that all groups had similar averages and more collagenosis occurred in the 525 nm green LED irradiation group than in the non-irradiation group.
Fibroblast growth and collagenosis have much to do with wound repair, and as such treated groups healed more quickly than the untreated group. Figure 2 shows images (200x magnification)of tissues for each group that had collagenosis. Specifically,Fig. 2(a) shows an image of collagen for the non-irradiation group indicating the presence of minute strands of collagen,while Fig. 2(b) is of the 525 nm green LED irradiation group,showing small and bigger collagen bundles.
To examine how fast tissues are regenerated, immunohistochemical staining was conducted to analyze expression of Proliferating Cell Nuclear Antigen (PCNA), focusing on the defect area under repair. It was determined that pan-Cytokeratin was positive when cytoplasm was stained with brown color and PCNA when the nucleus was stained. In order to compare between treatments, the most strongly stained 4 or 5 points of PCNA from each group were selected for imaging, analyzed with a computer measurement program, and the percentage of cells that were stained positive was calculated as a percent of the total number of cells counted. Table 4 shows the average percentage of PCNA in each group. No statistically significant difference was observed for PCNA expression between the 525 nm green LED irradiation group and non-irradiation group. We suspect that this result may have been obtained because each group went through the wound repair process from cell proliferation stage to collagenosis by the time of analysis. Specifically, PCNA expression relates to cell proliferation in wound areas, and thus it was not possible to clearly demonstrate that the extent of PCNA expression conformed to that of wound repair. Indeed, PCNA expression may be less when cells are quickly proliferating, thus leading to collagenosis.
[Table. 4.] Proliferating Cell Nuclear Antigen Staining (Mean ’ SD).

Proliferating Cell Nuclear Antigen Staining (Mean ’ SD).
In order to examine the effect of 525 nm green LED irradiation on wound healing, a 1 cm diameter round wound was excised from the back of experiment animals who were then given no irradiation therapy or 525 nm LED irradiation applied over 9 days for one hour per day. Following the therapy regimen, the repairing wound of each animal was subjected to histological examination.The features of the 525 nm green LED irradiation equipment were identified during animal testing and the conclusions are as follows:
The defect size of the wound in the experiment animal treated with 525 nm green LED irradiation was found to be decreased compared with the non irradiation group. Likewise, the rate at which the defect-size became smaller was increased in the 525 nm green LED irradiation group compared to the non-irradiation group in a statistically significantly manner (p’0.05).
Analysis of tissue with Masson’s trichrome stain was also carried out on tissues from irradiated and non-irradiated mice to evaluate the effect of 525 nm green LED irradiation. Our results showed that collagenosis was increased in the 525 nm green LED irradiation group compared with the non-irradiation group,which is consistent with the close relationship between collagenosis and wound repair and our observations of the rate of wound healing with 525 nm green LED irradiation.
Based on our results, we conclude that 525 nm green LED irradiation can induce cell proliferation to facilitate reepithelization during the process of wound healing. Lastly, it was also observed that cell proliferation was more active in the neighboring wound area following treatment with 525 nm green LED irradiation than in for the non-irradiation group in terms of decrease in defect size and collagenosis.