We have developed an environment-friendly etching process, an alternative to the dichromic acid etching process, as a pretreatment of acrylonitrile-butadiene-styrene (ABS) plastic for electroless plating. In order to plate ABS plastic in an electroless way, there should be fine holes on the surface of the ABS plastic to enhance mechanically the adhesion strength between the plastic surface and the plate. To make these holes, the surface was coated uniformly with dispersed chemical foaming agents in a mixture of environmentally friendly dispersant and solvent by the methods of dipping or direct application. The solvent seeps into just below the surface and distributes the chemical foaming agents uniformly beneath the surface. After drying off the surface, the surface was heated at a temperature well below the glass transition temperature of ABS plastic. By pyrolysis, the chemical foaming agents made fine holes on the surface. In order to discover optimum conditions for the formation of fine holes, the mixing ratio of the solvent, the dispersant and the chemical foaming agent were controlled. After the etching process, the surface was plated with nickel. We tested the adhesion strength between the ABS plastic and nickel plate by the cross-cutting method. The surface morphologies of the ABS plastic before and after the etching process were observed by means of a scanning electron microscope.
The Acrylonitrile-butadiene-styrene (ABS) plastic is a useful material for automobile parts, helmets, and parts of electronic devices, because it can be processed easily and has high thermal resistance and durability. To further improve durability and thermal resistance and to give metallic properties to the plastic surface, it is plated with metals such nickel [1, 2]. Nickel, a metal of silver-white color, has good malleability and ductility and is used to plate various plastics. The adhesion strength between the ABS plastic and the nickel is very important for the plating.In order to increase the adhesion strength, the surface of ABS plastic has been etched through the pretreatment process before the plating, using chemicals such as dichromic acid, fluoridation-hydrogen acid and hydrochloric acid [3] and this process has caused environmental problems. So, much research has been conducted with the aim of improving the etching process and has involved such techniques such as gaseous acid etching,glow discharge and plasma etching and ultrasound etching as alternatives [4-8]. However, none of the above methods have so far proved to be effective for use in the ABS manufacturing industry.
In this study, we introduce an environment-friendly etching process for ABS plastic using chemical foaming agents. We have confirmed the adhesion strength between the ABS plastic and nickel plate by the cross-cutting method. The surface morphologies of ABS plastic before and after the etching were observed using a scanning electron microscope (SEM).
Samples of ABS plastic with sizes of 30 mm × 40 mm × 1 mm were prepared for the etching and plating. The chemical foaming agent was MS140D from the Dongjinsemichem Corporation (Seoul, Korea). We formulated the environmentfriendly dispersant by mixing the chemical foaming agent with solvent (methanol was used as a solvent). To disperse the chemical foaming agent effectively, 24 kHz ultrasound was applied to the mixture for three minutes. A home-made infrared(IR) heater was used for the foaming in the temperature range 110-120°C.
At first, the sample surfaces were cleaned by a regular washing and rinsing process with methanol and deionized water in an ultrasonic bath. Then the samples were dipped in a mixture of an aqueous emulsion BK1036 purchased from the Bokwang Chemical Company (Jeongwangdong, Siheung, Kyunggido, Korea) and methanol was used as a thinner for less than a second. The volume ratio of the emulsion and the thinner was 1:20. Before the thinner was fully evaporated at room temperature, the samples were dipped in the dispersant prepared in advance for about a second. After the application of the dispersant, the samples were dried in air for 2-4 minutes; then, IR heat treatment was performed for a minute.
Three different dispersants were prepared as described above in using 20 mL methanol with chemical foaming agent quantities of 0.05 g, 0.2 g, and 0.6 g, respectively (hereafter we will denote A, B and C for the samples prepared with 0.05 g, 0.2 g and 0.6 g chemical foaming agents in the dispersants, respectively).
Three different dispersants were prepared as described above in using 20 mL methanol with chemical foaming agent quantities of 0.05 g, 0.2 g, and 0.6 g, respectively (hereafter we will denote A, B and C for the samples prepared with 0.05 g, 0.2 g and 0.6 g chemical foaming agents in the dispersants, respectively).
The most critical factor in the adhesion strength of the electroless plating is the degree of formation of holes on the sample surfaces. The holes act as anchor sites for the plated metal. In order to make these holes, we had used the chemical foaming agent that foams on the application of proper heat. To remove the residue of the foaming agent, the samples were treated in an ultrasonic bath for 5 minutes.
2.3 Electroless nickel plating
After the surfaces of ABS plastic samples were pretreated with the dispersants and IR heat treatment followed by the residue removal process, the samples were dipped in the catalyzing solution composed of palladium chloride and tin chloride at room temperature for 5 minutes, and then rinsed with deionized (DI)-water for 30 seconds.
To make metallic palladium on the sample surface by the reducing process, the samples were dipped in the accelerating solution solution at room temperature for 5 minutes, and then rinsed with DI-water for 30 seconds.
The metallic palladium on the sample surface acts as a reducer in the electroless plating process. The reducing agent does catalytic revitalization in the plating solution nickel sulfate and hypophosphate.The nickel plating was completed in a water bath at 80°C for 5 minutes, and after the plating process the samples were rinsed with DI-water for 30 seconds.
2.4 Measurement and observation
Surface morphologies of the samples were observed using the scanning electron microscope before and after IR-heat treatment of the chemical foaming agent distributed on the ABS plastic surfaces. The cross-cutting test was performed using 3M tape to check the adhesion strength of the plated nickel layer on the plastic surface after the electroless nickel plating [8]. For the ad-hesion test, we used the cross-cutting method with 3M tapes. In this case, the sample sizes were approximately 20 mm × 35 mm ×1 mm.
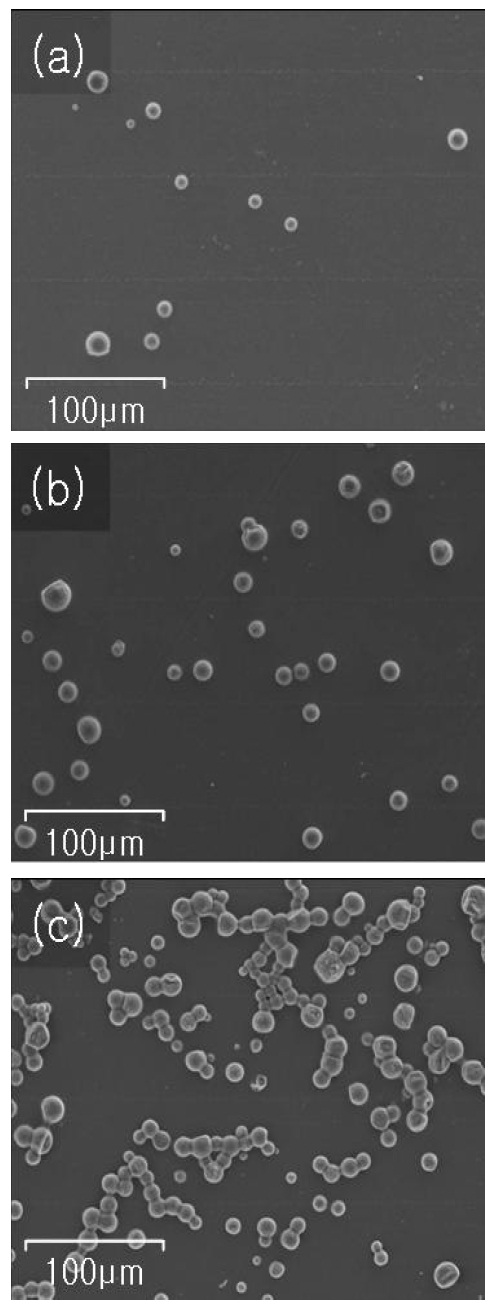
Figure 1 shows SEM images of the sample surfaces before IRheat treatment. As can be seen clearly in the figure, grains of the chemical foaming agents are distributed on the surfaces with certain size distributions in a range of 10-20 micrometer. Figures 1(a)-(c) show the results for samples A, B and C, respectively. For samples A and B, each grain has an almost spherical shape and is sited independently, which may be due to the dilute chemical foaming agent in the methanol. However for sample C, grains tend to group in curved chains. Also some of the chains are crossed each other.
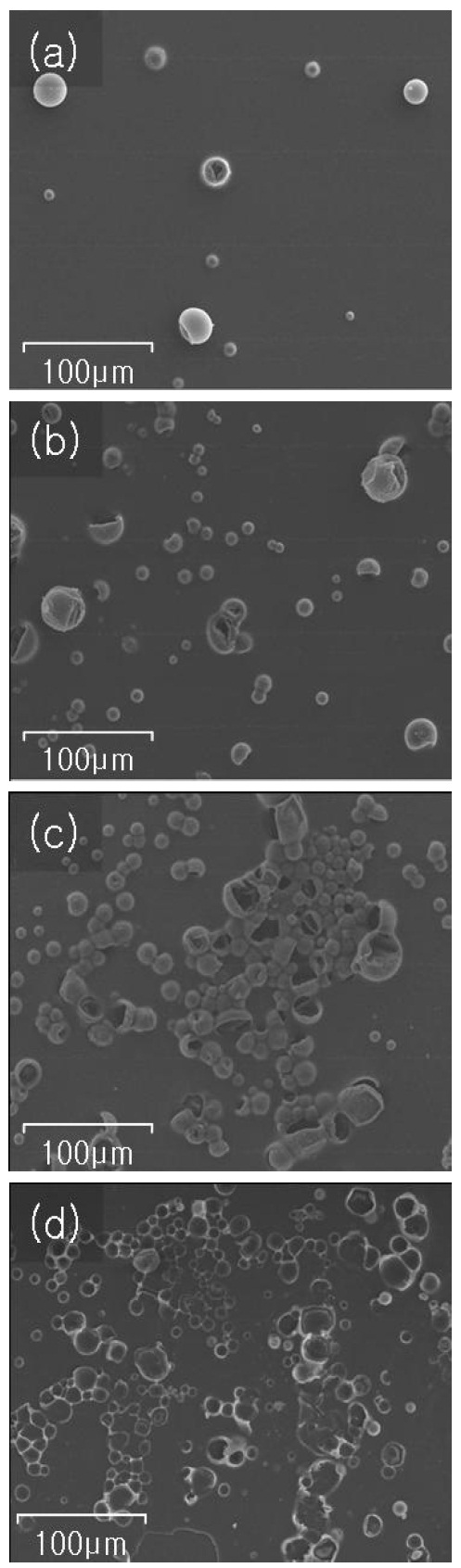
Surface images of the sample by SEM after IR-heat treatment are shown in Fig. 2. Figures 2(a)-(c) apply to samples A, B, and C,respectively. Due to the thermal foaming action of the agent, the grains burst out giving off nitrogen gas and then they collapsed and were crushed, making the sample surface rough, containing pores and holes. However, not all grains were burst; some of them remain inert as shown in the figures. Figure 2(d) shows SEM images of the sample surface after the agent residue on the sample C was removed by ultrasonic agitation in a bath. The residue removal process shows up an increased surface roughness and number of holes on the surface, which would in turn increase the anchor sites that improve the adhesion strength. As we described in the experimental section on the etching process,we have coated the sample firstly with the aqueous emulsion diluted in methanol and then the dispersant was applied before the emulsion was dried.
This process accelerates the formation of holes with the action of the foaming agent. The sites of the foaming agent grains erode the emulsion and holes grow as the emulsion is dried, so that there are holes left after the residual agent removal. Furthermore,the burst agent grains increase the surface roughness of the sample, which improves the adhesion strength.
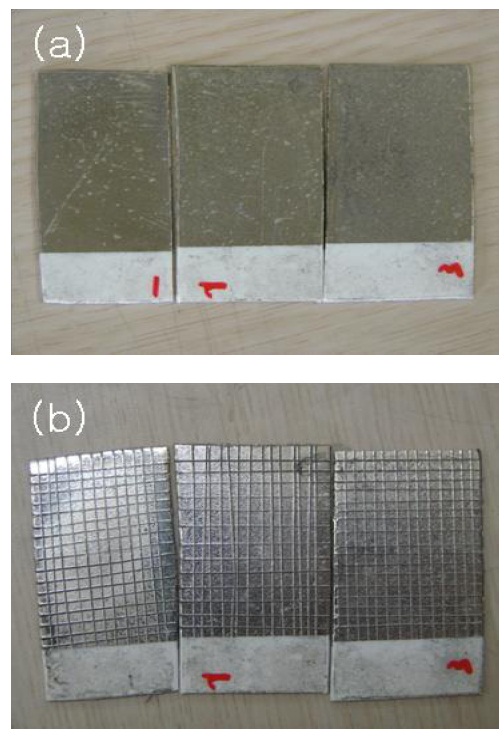
Figure 3 shows the results of the adhesion test by the crosscutting method with 3M tapes. In the figures, from the left to the right, the results refer to samples A to C respectively. All three samples showed good adhesion strength.
A new etching process for ABS plastic developed in this study by a chemical forming agent is simple and more environmentally friendly compared to the conventional one process. By the action of the foaming agent, surfaces with fine holes in the ABS plastic could be obtained. Adhesion between the nickel and the plastic was tested by the cross-cutting method, and it revealed good adhesion strength. The process developed in this study as an alternative to the dichromic acid etching process was found to be?environmentally friendly for the?electroless plating of the ABS plastic.