Phototrophic dinoflagellates are ubiquitous and sometimes dominate the abundance and biomass of plankton assemblages in marine environments (Porter et al. 1985, Hallegraeff 1993, Lindberg et al. 2005, Jeong et al. 2010a, 2013b). They can sometimes form dense blooms so called red tides or harmful algal blooms in marine ecosystem (Eppley and Horrison 1975, Burkholder et al. 2008, Kang et al. 2013, Park et al. 2013a). Dense blooms dominated by phototrophic dinoflagellates can upset the balance of food webs and cause great loss to the aquaculture and tourist industries in many countries (e.g., Park et al. 2013b). Furthermore, phototrophic dinoflagellates play diverse roles in marine planktonic food webs (Anderson et al. 2002, Yoo et al. 2009, Jeong et al. 2010b, Hansen 2011); they are primary producers (Tillmann et al. 2009), predators feeding on diverse prey items (Park et al. 2006, Berge et al. 2008, Yoo et al. 2010b, Jeong et al. 2012), and, in turn, act as prey for diverse predators (Jeong and Latz 1994, Jeong 1999, Tillmann 2004, Jeong et al. 2010b, Kim et al. 2013, Yoo et al. 2013b). Therefore, to understand the roles of phototrophic dinoflagellates in the food web, we must collate data on growth and mortality due to predation.
The phototrophic dinoflagellate Biecheleria cincta was previously named Woloszynskia cincta (Siano et al. 2009, Kang et al. 2011). However, this dinoflagellate has since been reclassified and moved from the genus Woloszynskia to the genus Biecheleria because it has genetic and morphological characteristics that more closely resemble Biecheleria, including an apical furrow apparatus formed by a single elongated narrow vesicle extending over the apex from the ventral to the dorsal side of the cell (Moestrup et al. 2009, Kang et al. 2011, Balzano et al. 2012, Luo et al. 2013). In addition, it has chloroplasts and eyespots that are formed by a stack of cisternae containing brick-like material (type E sensu) (Moestrup et al. 2009, Kang et al. 2011). The presence of this species has been reported in the coastal waters of Korea (Kang et al. 2011).
Recently, Kang et al. (2011) discovered that B. cincta, originally thought to be an exclusively phototrophic dinoflagellate, is a mixotrophic dinoflagellate; it feeds on diverse prey such as the haptophyte Isochrysis galbana, the cryptophytes Teleaulax sp. and Rhodomonas salina, the raphidophyte Heterosigma akashiwo, the euglenophyte Eutreptiella gymnastica, and the dinoflagellates Heterocapsa rotundata and Amphidinium carterae. However, to date, there have been no studies on the mortality of B. cincta due to predation. Grazing pressure sometimes plays an important role in controlling populations of phototrophic dinoflagellates (Watras et al. 1985, Turner 2006, Kang et al. 2013, Yoo et al. 2013a). Heterotrophic dinoflagellates and ciliates are the major components of heterotrophic protist communities (Sherr and Sherr 2007, Jeong et al. 2011, Yoo et al. 2013a). They are effective grazers on many phototrophic dinoflagellates (Eppley and Harrison 1975, Jeong et al. 2013a, Yoo et al. 2013b). Thus, to understand the population dynamics of B. cincta, the predator-prey relationships between B. cincta and common heterotrophic dinoflagellates and ciliates should be investigated.
We established a clonal culture of Biecheleria cincta isolated from Shiwha Bay, Korea in 2009. In the present study, using this culture we investigated feeding by five common heterotrophic dinoflagellates and one ciliate on this dinoflagellate; 1) we tested whether the common heterotrophic dinoflagellates Gyrodinium dominans, G. moestrupii, G. spirale, Oxyrrhis marina, and Polykrikos kofoidii and the ciliate Strobilidium sp. were able to feed on B. cincta. 2) We also measured the growth and/or ingestion rates of O. marina and Strobilidium sp. on B. cincta as a function of prey concentration. 3) In addition, the growth and ingestion rates were measured for G. dominans, G. moestrupii, G. spirale, and P. kofoidii at single prey concentrations at which these rates of O. marina and Strobilidium sp. were saturated. 4) Additionally, we compared the growth and ingestion rates of heterotrophic protists in the present study of B. cincta to those of other prey species reported in the literature.
The results of the present study provide a basis for understanding the interactions between B. cincta and heterotrophic protists and their population dynamics in marine planktonic food webs.
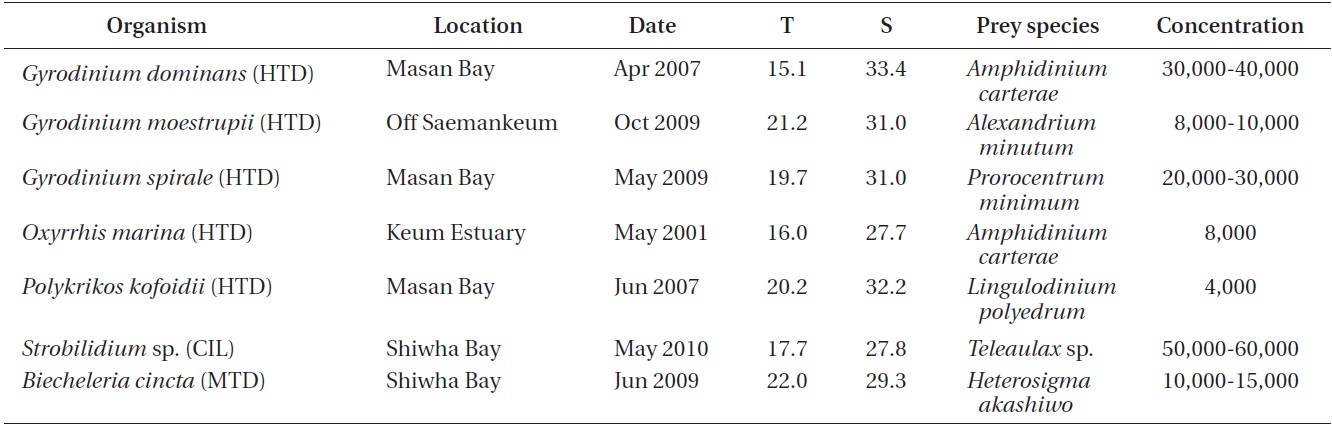
For isolation and culture of Biecheleria cincta (GenBank accession No. FR690459), plankton samples collected with water samplers were taken from Shiwha Bay, Korea during June 2009 when the water temperature and salinity were 22.0℃ and 29.3, respectively (Table 1). These samples were screened gently through a 154-μm Nitex mesh and placed in 6-well tissue culture plates. A clonal culture of B. cincta was established by two serial single cell isolations as described by Kang et al. (2011).
For the isolation and culture of the heterotrophic dinoflagellate predators Gyrodinium dominans, G. moestrupii, G. spirale, Oxyrrhis marina, and Polykrikos kofoidii, plankton samples collected with water samplers were taken from the coastal waters off Masan, Saemankeum, or Keum estuary, Korea in 2001-2009, and a clonal culture of each species was established by two serial single-cell isolations (Table 1).
For the isolation and culture of Strobilidium sp., plankton samples collected with water samplers were taken from a pier in Shiwha Bay, Korea, during May 2010 when the water temperature and salinity were 17.7℃ and 27.8, respectively (Table 1). A clonal culture of Strobilidium sp. (30-50 μm in cell length) was established by two serial single cell isolations as described by Jeong et al. (2008a).
The carbon contents for B. cincta (0.1 ng C per cell), the heterotrophic dinoflagellates, and the ciliate were estimated from the cell volume according to the methods described by Menden-Deuer and Lessard (2000). The cell volume of the predators was estimated using the methods described by Jeong et al. (2008b) and Jeong et al. (2008a) for O. marina and Strobilidium sp., respectively.
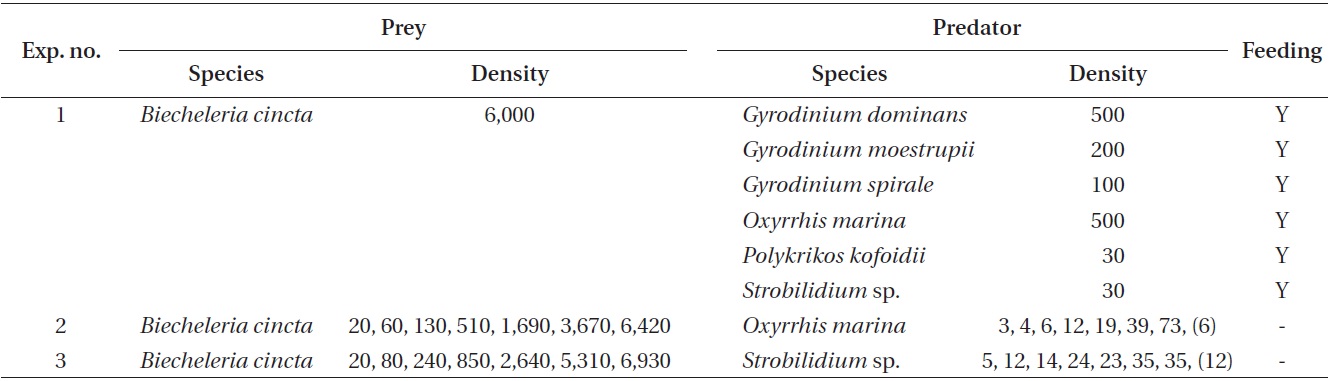
Experiment 1 was designed to investigate whether G. dominans, G. moestrupii, G. spirale, O. marina, P. kofoidii, and Strobilidium sp. were able to feed on B. cincta (Table 2). The concentrations of each predator species offered were similar in terms of carbon biomass.
Approximately 4.8 × 105 B. cincta cells were added to each of two 80 mL polycarbonate (PC) bottles containing G. dominans, G. moestrupii, G. spirale (100-500 cells mL-1), P. kofoidii (30 cells mL-1), and Strobilidium sp. (30 cells mL-1) (final B. cincta prey concentration = ca. 6,000 cells mL-1). One control bottle (without prey) was set up for each experiment. The bottles were placed on a plankton
wheel rotating at 0.9 rpm and incubated at 20℃ under an illumination of 20 μmol photons m-2 s-1 on a 14 : 10 h light-dark cycle.
Five milliliters aliquots were removed from each bottle after 1, 2, 6, 24, and 48-h incubation periods and then transferred into 6-well plate chambers (or slide glasses). Approximately 100 cells of predators at different stages of the feeding process in the plate chamber (or slide glasses) were observed under a dissecting microscope (or inverted microscope) at a magnification of ×20-90 (or ×100-630) to determine whether the predators were able to feed on B. cincta. Cells from those predators that contained ingested B. cincta cells were photographed using a digital camera (Zeiss AxioCam HRc5; Carl Zeiss Ltd., Gottingen, Germany) on the microscope at a magnification of ×400-630.
Experiments 2 and 3 were designed to measure the growth, ingestion, and clearance rates of O. marina and Strobilidium sp. as a function of the prey concentrations when fed on B. cincta (Table 2).
A dense culture of ~15,000 cells mL?1 of B. cincta grown mixotrophically on the raphidophyte Heterosigma akashiwo in the f/2 medium (Guillard and Ryther 1962, Kang et al. 2011) under the illumination of 20 μmol photons m-2 s-1 on a 14 : 10 h light : dark cycle was transferred to a 250-mL PC bottle containing the f/2 medium wherein H. akashiwo was undetectable. This culture was maintained in the f/2 medium for 2 d under the illumination of 20 μmol photons m-2 s-1 on a 14 : 10 h light : dark cycle and then transferred to another 250-mL PC bottle containing filtered seawater. Three 1-mL aliquots from the bottle were examined using a light microscope to determine the concentration of B. cincta cells, and the cultures were then used in further experiments.
Furthermore, dense cultures of O. marina (or Strobilidium sp.) growing on algal prey were transferred into 250-mL PC bottles containing filtered seawater. The bottles were filled to capacity with freshly filtered seawater, capped, and placed on plankton wheels rotating at 0.9 rpm and incubated at 20℃ under the illumination of 20 mol photons m-2 s-1 on a 14 : 10 h light : dark cycle.
For each experiment, the initial concentrations of O. marina (or Strobilidium sp.) and B. cincta were established using an autopipette to deliver predetermined volumes of known cell concentrations to the bottles. Triplicate 42-mL PC experimental bottles (mixtures of predator and prey) and triplicate control bottles (prey only) were set up for each predator-prey combination. Triplicate control bottles containing only O. marina (or Strobilidium sp.) were also established for a single predator concentration. All the bottles were filled to capacity with freshly filtered seawater and capped. To determine the actual predator and prey densities at the beginning of the experiment, a 5-mL aliquot was removed from each bottle, fixed with 5% Lugol’s solution, and then examined under a light microscope to determine predator and prey abundance by enumerating the cells in three 1-mL Sedgwick-Rafter chambers (SRCs). The bottles were refilled to capacity with freshly filtered seawater, capped, and placed on rotating wheels under the conditions described earlier in this section. Dilution of cultures associated with the refilling of bottles was taken into consideration when calculating growth and ingestion rates. A 10-mL aliquot was taken from each bottle after a 48-h incubation period and fixed with 5% Lugol’s solution, and the abundance of O. marina (or Strobilidium sp.) and B. cincta were determined by counting all or >200 cells in three 1-mL SRCs. Prior to taking the subsamples, the conditions of O. marina (or Strobilidium sp.) and its prey were assessed using a dissecting microscope, as described earlier in this section.
The specific growth rate of O. marina (or Strobilidium sp.), μ (d-1) was calculated as follows:
, where G0 and Gt are the concentration of O. marina (or Strobilidium sp.) at time (t) 0 and 2 d, respectively.
Data for O. marina (or Strobilidium sp.) growth rates were fitted to a modified Michaelis-Menten equation:
, where μmax = the maximum growth rate (d-1), x = prey concentration (cells mL-1 or ng C mL-1), x' = threshold prey concentration (the prey concentration where μ = 0), and KGR = the prey concentration sustaining 1/2 μmax. Data were iteratively fitted to the model using DeltaGraph (Delta Point Inc., Monterey, CA, USA).
Ingestion and clearance rates were calculated using the equations of Frost (1972) and Heinbokel (1978). The incubation time for calculating ingestion and clearance rates was the same as that for estimating growth rate. Data for O. marina (or Strobilidium sp.) ingestion rates (IR, cells predator-1 d-1 or ng C predator-1 d-1) were fitted to a modified Michaelis-Menten equation:
, where Imax = the maximum ingestion rate (cells predator-1 d-1 or ng C predator-1 d-1), x = prey concentration (cells mL-1 or ng C mL-1), and KIR = the prey concentration sustaining 1/2 Imax.
Experiment 4 was designed to compare the growth and ingestion rates of G. dominans, G. moestrupii, G. spirale, and P. kofoidii when B. cincta was provided at a single prey concentration (Table 3). Growth and ingestion rates of O. marina and Strobilidium sp. at single prey concentrations were obtained in Experiment 2 and 3.
The B. cincta culture was prepared as described earlier in this section. In addition, G. dominans, G. moestrupii, G. spirale, and P. kofoidii were cultured in the same manner as described earlier in this section.
The initial concentrations of G. dominans (or another predator) and B. cincta were established using an autopipette to deliver predetermined volumes of known cell concentrations to the bottles. Triplicate 42-mL PC experimental bottles containing mixtures of G. dominans (or another predator) and B. cincta, triplicate prey control bottles containing B. cincta only, and triplicate predator control bottles containing only G. dominans (or another predator) were set up for B. cincta. Next, 5 mL of the f/2 medium was added to all the bottles, which were then filled to capacity with freshly filtered seawater and capped. To determine the predator and prey concentrations at the beginning of the experiment and the prey concentrations after 2 d, a 5-mL aliquot was removed from each bottle and fixed with 5% (v/v) Lugol’s solution; then, all or >200 predator and prey cells from three 1-mL SRCs were enumerated. Prior to taking subsamples, the conditions of G. dominans (or other predators) and B. cincta were assessed using a dissecting microscope. The bottles were, again, filled to capacity with freshly filtered seawater, capped, and placed on a rotating wheel at 0.9 rpm at 20℃ under the illumination of 20 μmol photons m-2 s-1 on a 14 : 10 h light : dark cycle. The dilution of the cultures associated with the refilling of bottles was taken into consideration when calculating the growth and ingestion rates.
The growth and ingestion rates were measured in the same manner as described for Experiments 2 and 3.
GGE, defined as grazer biomass produced (+) or lost (?) per prey biomass ingested, was calculated from the estimates of carbon contents per cell based on the cell volume for each mean prey concentration.
It was observed that G. dominans, G. moestrupii, G. spirale, O. marina, P. kofoidii, and Strobilidium sp. fed on B. cincta (Table 2, Fig. 1). All predators in the present study fed on prey by engulfing the prey cells.
B. cincta clearly supported positive growth rates for O. marina, G. dominans, and Strobilidium sp. but did not support the growth of G. moestrupii, G. spirale, or P. kofoidii.
The specific growth rates of O. marina on B. cincta increased rapidly with increasing mean prey concentration -1 (120 cells mL-1), but became saturated or slowly increased at higher concentrations (Fig. 2). When the data were fitted to Eq. (2), the maximum specific growth rates of O. marina was 0.49 d-1. The feeding threshold prey concentration for the growth of O. marina was 1.4 ng C mL-1 (14 cells mL-1).
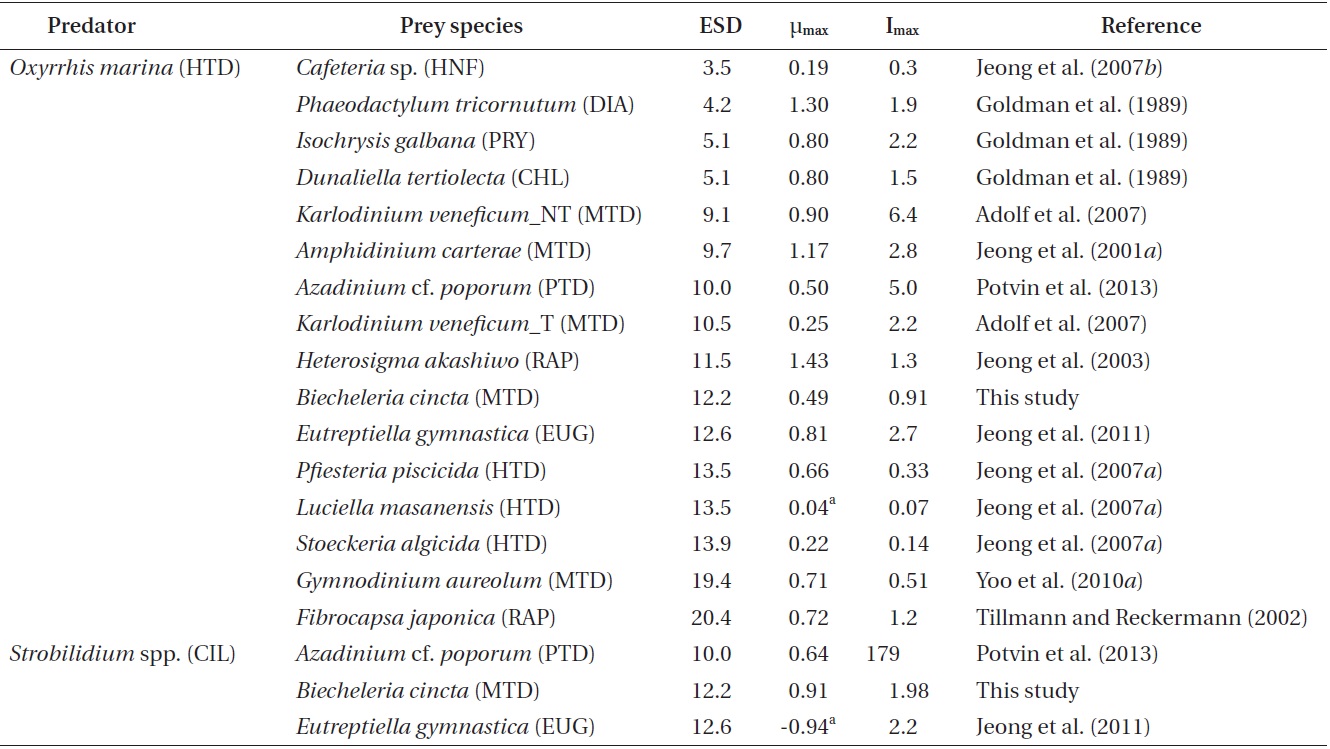
The specific growth rates of Strobilidium sp. on B. cincta increased rapidly with increasing mean prey concentration -1 (710 cells mL-1), but became slowly increased at higher concentrations (Fig. 3). When the data were fitted to Eq. (2), the maximum specific growth rates of Strobilidium sp. was 0.91 d-1. The feeding threshold prey concentration for the growth of Strobilidium sp. was 11.8 ng C mL-1 (118 cells mL-1).
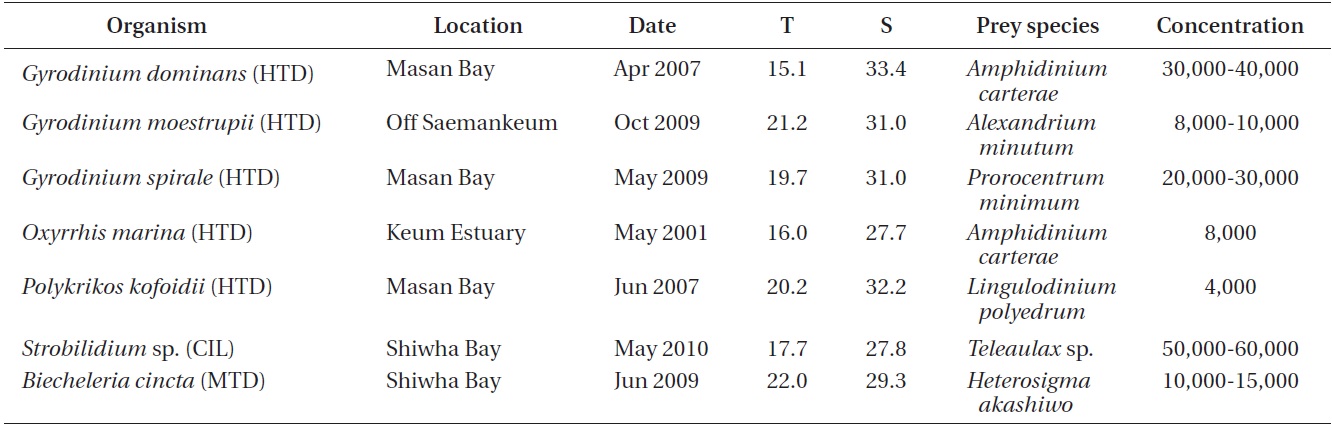
The ingestion rates of O. marina on B. cincta increased rapidly with increasing mean prey concentration -1 (450 cells mL-1), but became saturated or slowly increased at higher concentrations (Fig. 4). When the data were fitted to Eq. (3), the maximum ingestion rates of O. marina was 0.35 ng C predator-1 d-1 (3.5 cells predator-1 d-1). The maximum clearance rate of O. marina was 1.47 μL
predator-1 h-1. GGEs of O. marina on B. cincta at prey concentrations where the ingestion rates were saturated were 45-53% (Table 4).
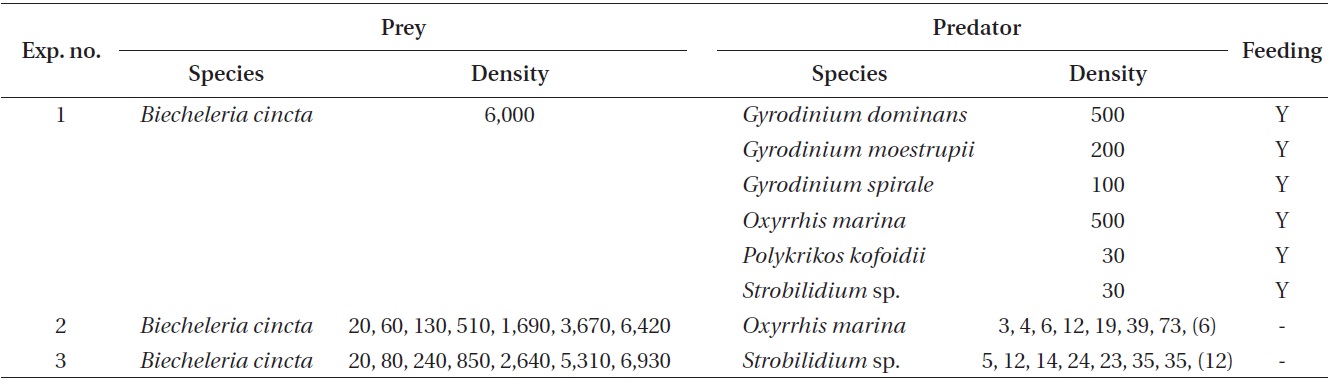
The ingestion rates of Strobilidium sp. on B. cincta increased rapidly with increasing mean prey concentration -1 (2,360 cells mL-1), but became slowly increased at higher concentrations (Fig. 5). When the data were fitted to Eq. (3), the maximum ingestion rates of Strobilidium sp. was 2.0 ng C predator-1 d-1 (20.0 cells predator-1 d-1). The maximum clearance rate of Strobilidium sp. was 1.72 μl predator-1 h-1. GGEs of Strobilidium sp. on B. cincta at prey concentrations where the ingestion rates increased slowly were 25-32% (Table 4).
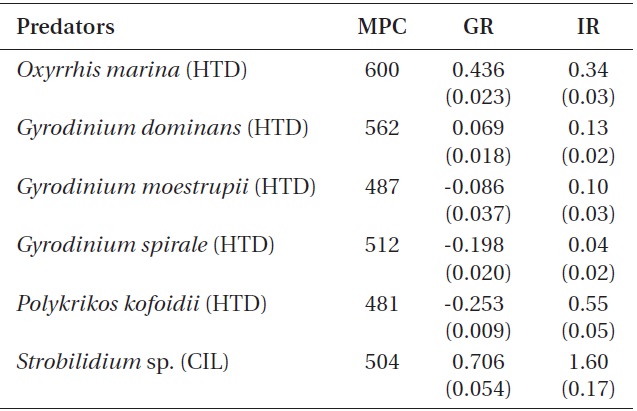
When the mean prey concentrations were 480-600 ng C mL-1, the specific growth rate of Strobilidium sp. (0.71 d-1) on B. cincta was significantly higher than that of O. marina (0.44 d-1) or G. dominans (0.07 d-1) (p < 0.01, two-tailed t-test). However, the growth rates of G. moestrupii, G. spirale, and P. kofoidii were negative (Table 3).
The ingestion rate of Strobilidium sp. (1.60 ng C predator-1 d-1)
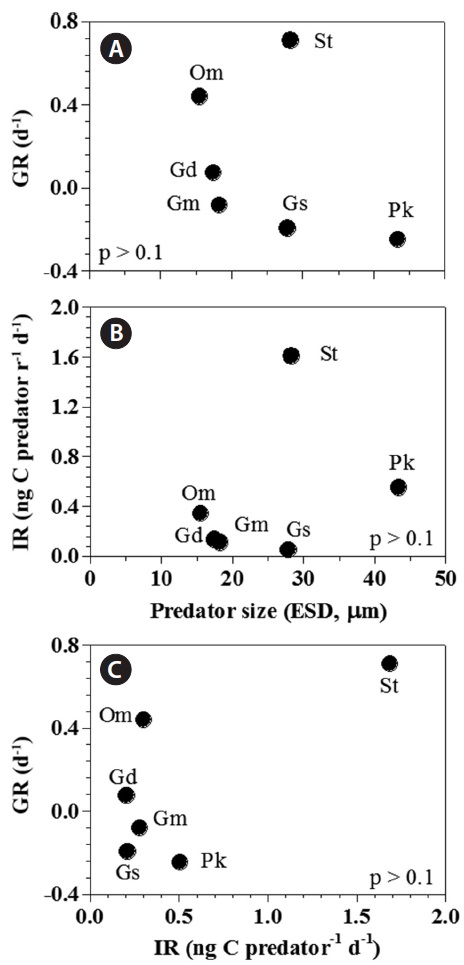
[Fig. 6.] Growth (GR) and ingestion rates (IR) of heterotrophic dinoflagellates and the ciliate on the mixotrophic dinoflagellate Biecheleria cincta as a single prey concentration where the growth and ingestion rates of Oxyrrhis marina and Strobilidium sp. were saturated. GR (A) and IR (B) of the predators on B. cincta as a function of predator size (equivalent spherical diameter, ESD, μm). (C) The GR of predators on B. cincta as a function of the IR (as shown in Table 3). The p-values in (A), (B), and (C) were all p > 0.1 (linear regression ANOVA). Gd, Gyrodinium dominans; Gm, Gyrodinium moestrupii; Gs, Gyrodinium spirale; Om, O. marina; Pk, Polykrikos kofoidii; St, Strobilidium sp.
on B. cincta was significantly higher than that of P. kofoidii (0.55 ng C predator -1 d-1), O. marina (0.34 ng C predator -1 d-1), G. dominans (0.13 ng C predator -1 d-1), G. moestrupii (0.10 ng C predator -1 d-1), or G. spirale (0.04 ng C predator -1 d-1) (p < 0.01, two-tailed t-test).
Both growth and ingestion rates of the heterotrophic protists feeding on B. cincta in the present study were not significantly correlated with the predators’ equivalent spherical diameter (p > 0.1, linear regression analysis of variance [ANOVA]) (Fig. 6A & B). Moreover, the growth rates were not significantly correlated with ingestion rates (p > 0.1, ANOVA) (Fig. 6C).
To the best of our knowledge, this study is the first report on feeding by heterotrophic protistan predators on B. cincta. All heterotrophic protistan predators investigated in the present study were able to feed on B. cincta by engulfing the cells. These heterotrophic protists commonly occur in many marine environments (Goldman et al. 1989, Yoo et al. 2010a, Jeong et al. 2011, Yoon et al. 2012). Thus, heterotrophic protists should be considered predators of B. cincta in marine food webs.
O. marina, G. dominans, and Strobilidium sp. exhibited positive growth rates when feeding on B. cincta but G. moestrupii, G. spirale, and P. kofoidii did not. Thus, during blooms dominated by B. cincta, O. marina, G. dominans, and Strobilidium sp. are likely to be abundant, while G. moestrupii, G. spirale, and P. kofoidii may not be present. B. cincta can be a critical prey for selecting dominant species among heterotrophic protistan communities.
The growth rates for G. moestrupii, G. spirale, and P. kofoidii feeding on B. cincta were negative, while those for O. marina or Strobilidium sp. were relatively high (Table 3). For G. moestrupii, G. spirale, and P. kofoidii feeding on B. cincta, their ingestion rates (0.10, 0.04, and 0.55 ng C predator-1 d-1, respectively) were much lower than their carbon contents (0.4, 1.3, and 4.2 ng C cell-1, respectively) (Jeong et al. 2001b, Kim and Jeong 2004, Yoo et al. 2013b). Thus, low ingestion rates for G. moestrupii, G. spirale, and P. kofoidii on B. cincta are likely responsible for their negative growth rates. However, growth rates for G. moestrupii, G. spirale, and P. kofoidii are high when feeding on algal prey (Jeong et al. 2001b, Kim and Jeong 2004, Yoo et al. 2013b). The maximum growth rates for G. moestrupii, G. spirale, and P. kofoidii when feeding on optimal prey (e.g., Alexandrium minutum, Prorocentrum minimum, and Gymnodinium catenatum) are as high as 1.60, 1.13, and 1.12 d-1, respectively (Jeong et al. 2001b, Kim and Jeong 2004, Yoo et al. 2013b). We assume that the ecological niches of G. moestrupii, G. spirale, and P. kofoidii may be different from those of O. marina or Strobilidium sp., and competition among these protistan grazers might reduce when feeding on certain prey.
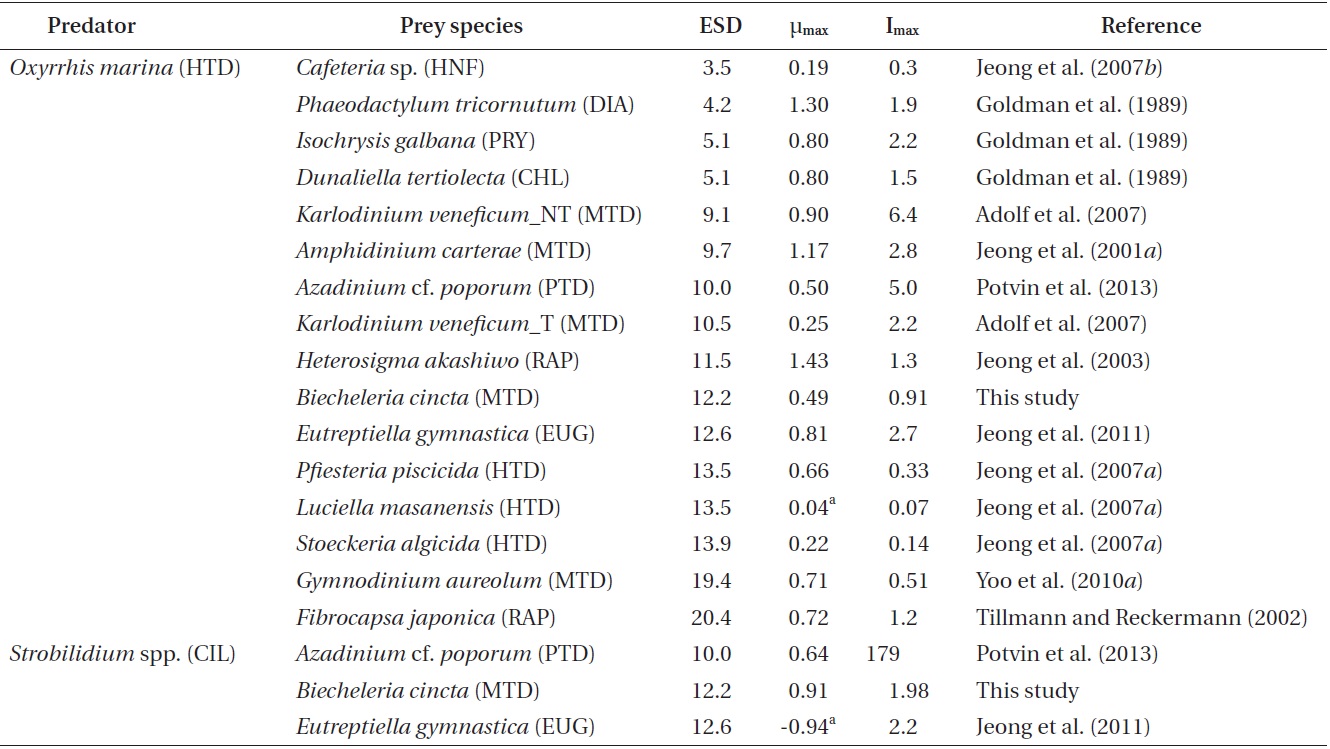
Among the maximum growth (μmax) and ingestion rates (Imax) of O. marina feeing on diverse prey items, the μmax of O. marina on B. cincta is similar than that on Azadinium cf. poporum (Table 5). However, the Imax of O. marina on B. cincta is lower than that on A. cf. poporum (Potvin et al. 2013). Therefore, the nutritional value of B. cincta for growth of O. marina may be greater than that of A. cf. poporum. The μmax of O. marina feeding on B. cincta is lower than that on the other algal prey species except a toxic strain of Karlodinium veneficum, but higher than that on the heterotrophic nanoflagellate Cafeteria sp. and the heterotrophic dinoflagellates Luciella masanensis and Stoeckeria algicida (Table 5). Therefore, B. cincta is a better prey item for O. marina than these heterotrophic nanoflagellate and heterotrophic dinoflagellates, but less favorable prey than the other algal prey species, except K. veneficum. The Imax of O. marina feeding on B. cincta is lower than that on the other algal prey species except the mixotrophic dinoflagellate Gymnodinium aureolum, but higher than that on Cafeteria sp., Pfiesteria piscicida, L. masanensis, and S. algicida (Table 5). Therefore, the lower ingestion rate of O. marina feeding on B. cincta than that on the other algal prey species except one species may be responsible for its lower growth rates, but the higher ingestion
rate of O. marina feeding on B. cincta than that on the heterotrophic nanoflagellate and heterotrophic dinoflagellates may be responsible for its higher growth rates.
O. marina may capture and ingest B. cincta with more difficulty than the other algal prey, except some unpalatable ones, but more easily than heterotrophic nanoflagellate and dinoflagellates. Both the μmax and Imax of O. marina feeding on diverse prey species, including B. cincta, were not significantly correlated with the prey’s equivalent spherical diameter (p > 0.1, ANOVA). Moreover, the μmax of O. marina feeding on diverse prey species was not significantly correlated with the Imax (p > 0.1, ANOVA). Therefore, for O. marina, the nutritional value of the different prey species, including B. cincta, may differ.
The μmax of Strobilidium sp. on B. cincta is higher than that on A. cf. poporum and the euglenophyte Eutreptiella gymnastica, although the Imax of Strobilidium sp. on B. cincta is comparable to or lower than that on A. cf. poporum and E. gymnastica (Table 5). Therefore, for Strobilidium sp., B. cincta may have higher nutritional value than A. cf. poporum or E. gymnastica.
Both growth and ingestion rates of O. marina and Strobilidium sp. on B. cincta are affected by prey concentrations. The threshold prey concentration for growth of O. marina on B. cincta (1.4 ng C mL-1) was lower than that for the growth rate of Strobilidium sp. on the same prey (11.8 ng C mL-1). Therefore, O. marina is likely to survive at low B. cincta concentrations but Strobilidium sp. is not. The KGR (the prey concentration sustaining 1/2 μmax) of 5.7 ng C mL-1 for O. marina feeding on B. cincta was also lower than that of Strobilidium sp. (34.8 ng C mL-1) feeding on the same algal prey. Thus, O. marina is likely to grow rapidly at low B. cincta concentrations but Strobilidium sp. would not. Additionally, the KIR (the prey concentration sustaining 1/2 Imax) of 9.2 ng C mL-1 for O. marina feeding on B. cincta was also lower than that of Strobilidium sp. (62.2 ng C mL-1) feeding on the same algal prey. Thus, these results indicate that, at low prey concentrations, the growth and ingestion rates of O. marina would respond more readily to changes in prey concentrations than those of Strobilidium sp.
We could not estimate the grazing impact by O. marina and Strobilidium sp. on B. cincta in this study because data on the abundance of B. cincta, O. marina, and Strobilidium sp. are not available. Therefore, to understand the population dynamics of B. cincta and heterotrophic protists and their interactions, the abundance of B. cincta and its predators in natural environments need to be quantified.




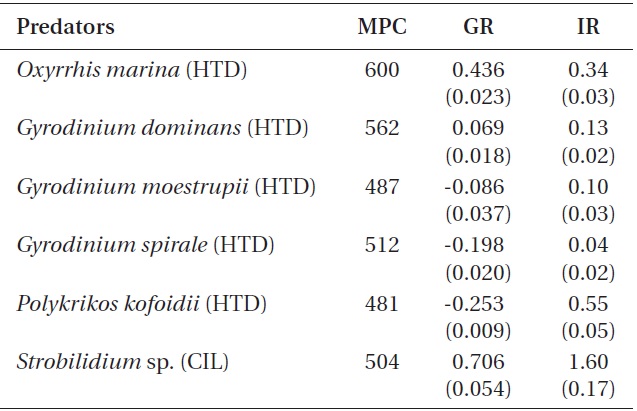









![Specific growth rates of the heterotrophic dinoflagellate
Oxyrrhis marina on the mixotrophic dinoflagellate Biecheleria cincta
as a function of mean prey concentration (x). Symbols represent
treatment means ± 1 SE. The curves are fitted according to the
Michaelis-Menten equation [Eq. (2)] using all treatments in the
experiment. Growth rate (d-1) = 0.492{(x - 1.38)/[5.67 + (x - 1.38)]},
r2 = 0.843.](http://oak.go.kr/repository/journal/12779/JORHBK_2013_v28n4_343_f002.jpg)
![Specific growth rates of the ciliate Strobilidium sp. on the
mixotrophic dinoflagellate Biecheleria cincta as a function of mean
prey concentration (x). Symbols represent treatment means ± 1 SE.
The curves are fitted according to the Michaelis-Menten equation [Eq.
(2)] using all treatments in the experiment. Growth rate (d-1) = 0.910
{(x - 11.8)/[34.8 + (x - 11.8)]}, r2 = 0.911.](http://oak.go.kr/repository/journal/12779/JORHBK_2013_v28n4_343_f003.jpg)
![Ingestion rates of the heterotrophic dinoflagellate Oxyrrhis
marina on the mixotrophic dinoflagellate Biecheleria cincta as
a function of mean prey concentration (x). Symbols represent
treatment means ± 1 SE. The curves are fitted according to the
Michaelis-Menten equation [Eq. (3)] using all treatments in the
experiment. Ingestion rate (ng C predator-1 d-1) = 0.35[x/(9.22 + x)],
r2 = 0.777.](http://oak.go.kr/repository/journal/12779/JORHBK_2013_v28n4_343_f004.jpg)
![Ingestion rates of the ciliate Strobilidium sp. on the mixotrophic
dinoflagellate Biecheleria cincta as a function of mean prey concentration
(x). Symbols represent treatment means ± 1 SE. The curves
are fitted according to the Michaelis-Menten equation [Eq. (3)] using
all treatments in the experiment. Ingestion rate (ng C predator-1 d-1) =
1.98 [x/(62.2 + x)], r2 = 0.884.](http://oak.go.kr/repository/journal/12779/JORHBK_2013_v28n4_343_f005.jpg)