There is increasing interest in the relationships between marine bacteria and red tide organisms. Some bacteria are known to kill red tide organisms, and may be responsible for accelerating the termination of red tides. Thus, certain algicidal bacteria have been proposed for the control of red tides. Meanwhile, many red tide organisms are known to feed on marine bacteria. The roles of marine bacteria and red tide organisms are therefore reversible. In Korean waters, the killing of red tide organisms by algicidal bacteria, and also the feeding of red tide organisms on marine bacteria have been extensively investigated. The findings of such studies may influence the conventional view of red tide dynamics, and also planktonic food webs. Here, we review the species and concentrations of algicidal bacteria that kill red tide organisms in Korean waters, as well as the ingestion rate and grazing impact of red tide organisms on marine bacteria. Furthermore, we offer an insight into the ecological roles of these 2 components in marine planktonic food webs.
Red tides―discoloration of the surface of the sea due to the blooms of plankton―constitute one of the most important environmental issues globally. By altering the balance of food webs and causing large-scale mortalities of fish and shellfish, red tides often lead to considerable losses in the aquaculture and tourist industries (Whyte et al. 2001, Curtiss et al. 2008, Richlen et al. 2010, Jeong and Kang 2013, Park et al. 2013
Several investigations have revealed that certain bacteria kill red tide organisms, thereby playing an important role in the decline of red tides (Imai et al. 1993, 2001, Doucette et al. 1998, Salomon and Imai 2006). Thus, the killing of red tide organisms by algicidal bacteria has been extensively studied (Fukami et al. 1992, Mayali and Doucette 2002). Meanwhile, in the last 2 decades, many red tide organisms, including phototrophic dinoflagellates and raphidophytes, have been shown to feed on bacteria (Nygaard and Tobiesen 1993, Seong et al. 2006, Jeong et al. 2010
In Korea, red tides have led to considerable losses in the aquaculture industries (Park et al. 2013
Here, we review the species and concentrations of algicidal bacteria that kill red tide organisms in Korean waters, as well as the ingestion rates and grazing impact of red tide organisms on bacteria. Furthermore, we examine the ecological significance of the interactions between these 2 components of marine environments.
BACTERIA AS KILLERS OF RED TIDE ORGANISMS IN KOREAN WATERS
>
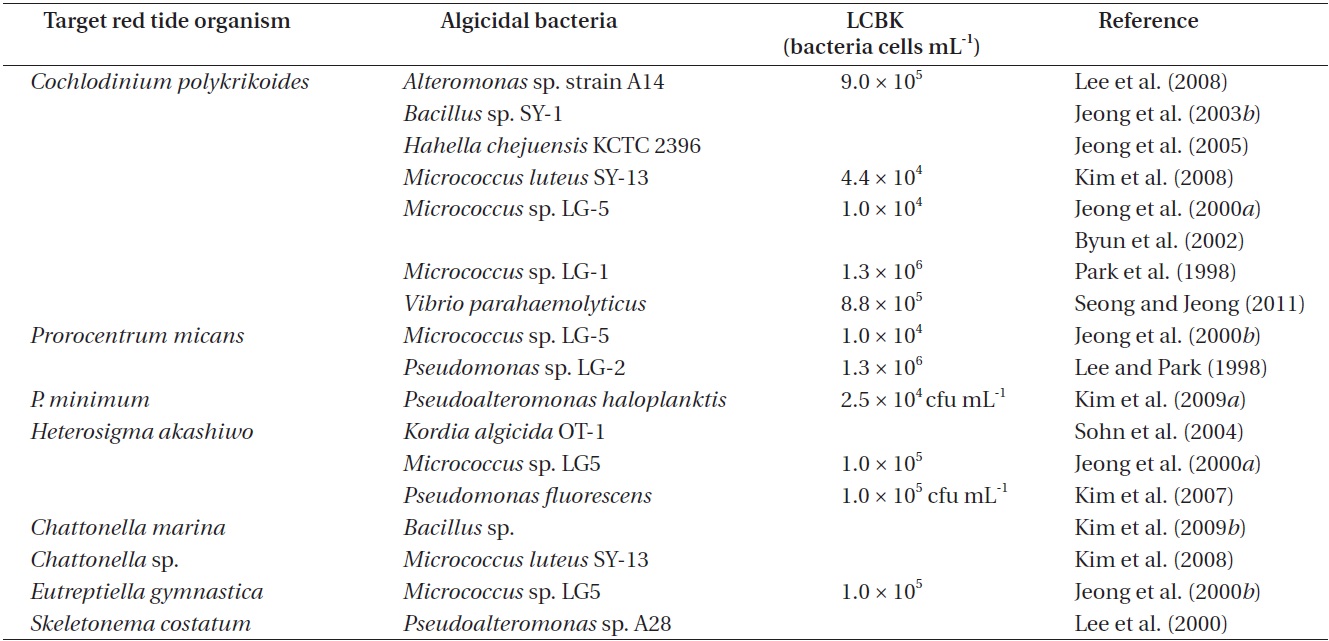
Species of algicidal bacteria isolated from Korean waters
Many bacteria are known to kill red tide organisms in Korean waters (Table 1). In particular, algicidal bacteria that kill the mixotrophic dinoflagellate
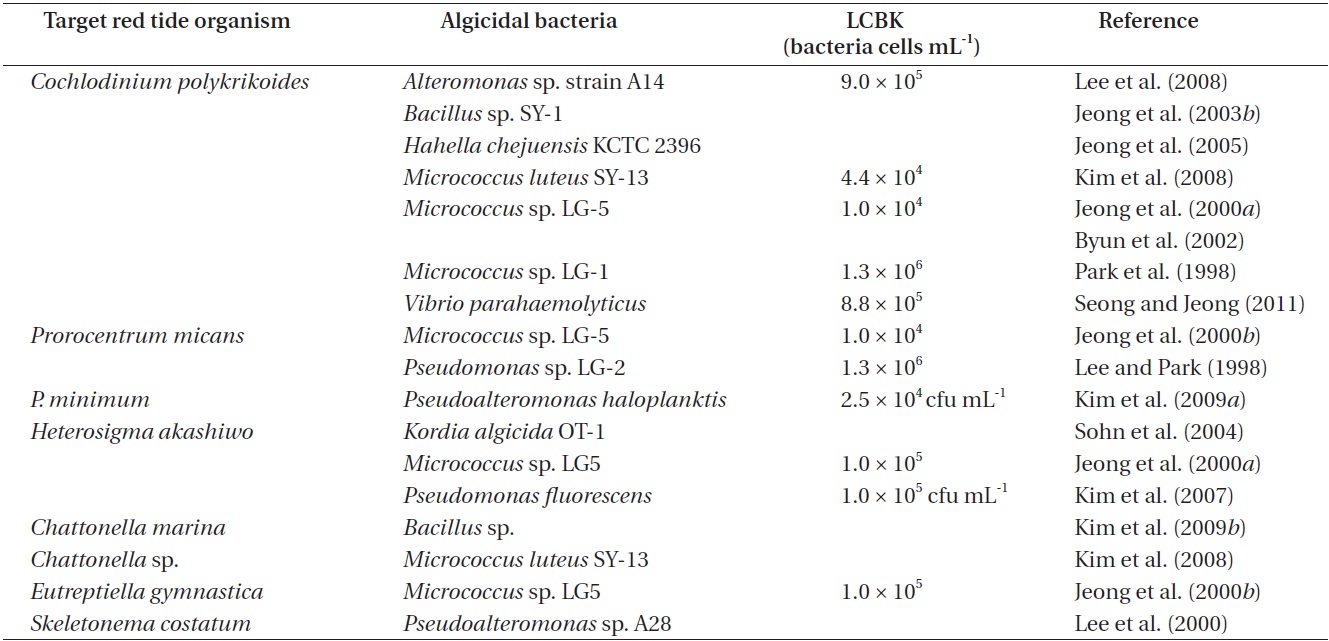
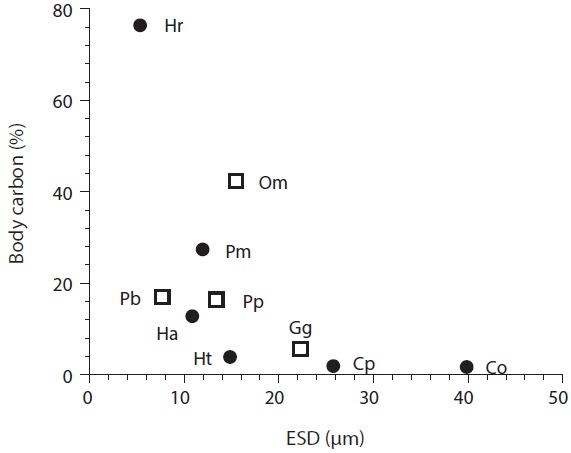
Algicidal bacteria and target red tide organisms isolated from the Korean waters, and lowest concentrations of algicidal bacteria required to kill red tide organisms (LCBK)
bacteria include
The bacterium
On the basis the results of laboratory and field experiments, methods to control red tides using mass-cultured algicidal bacteria have been developed (e.g., Kim et al. 2009
The lowest concentrations of algicidal bacteria required to kill target red tide organisms (LCBK) differ depending on the species of bacteria and red tide organisms (Table 1). For example, the LCBK of
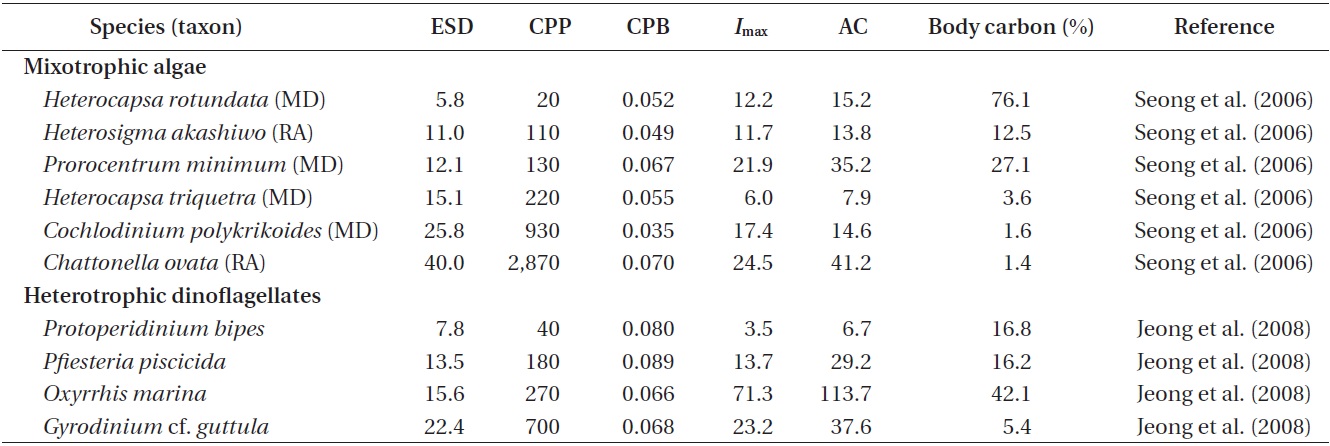
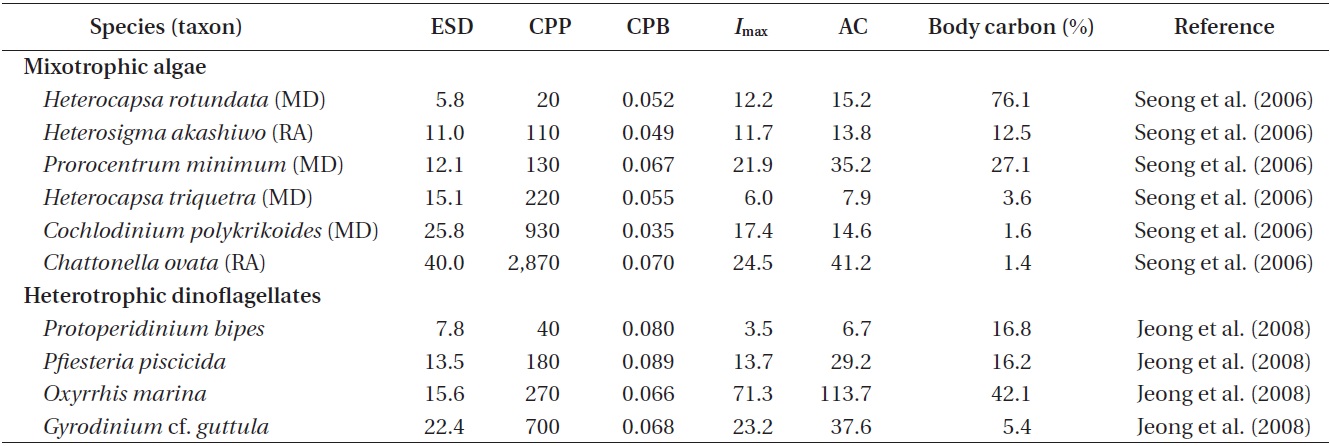
Ingestion rates and carbon acquisition of mixotrophic and heterotrophic predators of marine bacteria
using the control methods in natural environments, the LCBK must be determined.
>
Mesocosms testing of algicidal bacteria for killing red tide organisms in Korean waters
The use of algicidal bacteria to kill red tide organisms has frequently been investigated by means of field mesocosms (Kim et al. 2009
BACTERIA AS PREY FOR RED TIDE ORGANISMS IN KOREAN WATERS
Mixotrophic red tide dinoflagellates. Many mixotrophic red tide dinoflagellates isolated from Korean waters are known to feed on bacteria (Table 2). For example,
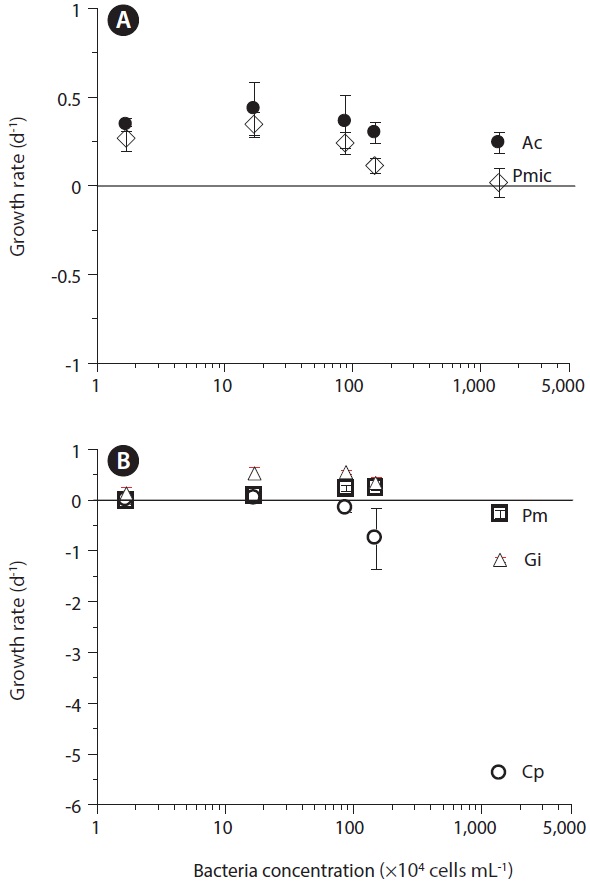
Ingestion and clearance rates measured in the laboratory. Seong et al. (2006) reported that an increase in the initial bacterial prey concentration to ca. 5 × 106 to 10 × 106 cells mL-1 led to a rapid increase in the ingestion rates by
The maximum bacterial clearance rates by red tide algae were 1.0-2.3 nL alga-1 h-1 for
Carbon acquisition from bacterial prey. Seong et al. (2006) reported that the smallest red tide alga,
Ingestion rates measured in the field. Seong et al. (2006) reported that the mean ingestion rates of natural bacterial populations by mixotrophic red tide dinoflagellates in Korean coastal waters ranged from 1.2 to 20.6 bacteria alga-1 h-1 for
>
Mixotrophic red tide raphidophytes
The mixotrophic red tide raphidophytes
The maximum ingestion rates of
Seong et al. (2006) reported that the ingestion rate of
>
Heterotrophic dinoflagellates
Jeong et al. (2006) and Yoo et al. (2013) reported that the abundance of the heterotrophic dinoflagellate
RECIPROCAL PREDATION BETWEEN BACTERIA AND RED TIDE ORGANISMS
The pathogenic bacterium
On the basis of data derived from studies of bacterial
feeding by red tide organisms, and killing of red tide organisms by algicidal bacteria, a hub of each red tide organism can be drawn (Jeong et al. 2010
ECOLOGICAL SIGNIFICANCE OF THE INTERACTIONS BETWEEN BACTERIA AND RED TIDE ORGANISMS
Taken together, the results of previous studies on the interactions between heterotrophic bacteria and red tide organisms indicate the following roles of each marine component in the dynamics of the other: 1) bacteria can be killers of red tide organisms; 2) bacteria can clear the body of senescent red tide organisms, by accelerating the decline of a red tide and decomposing the red tide organisms; 3) during some red tides, dominant red tide organisms may be the most effective predators of marine bacteria among protistan predators; and 4) bacteria may be too small to be ingested by filter-feeding copepods, whereas many red tide organisms are ingested by the copepods. Red tide organisms may therefore represent a link between bacteria and some zooplankters, which are unable directly to ingest bacteria.
Thus, bacteria may play diverse roles in red tide dynamics, and may even be critical factors affecting the abundance of red tide organisms in Korean waters.
Marine heterotrophic bacteria and red tide organisms can act as predators and / or prey in Korean waters. Furthermore, their roles are reversible at any time. Thus, these 2 components may co-exist by cycling materials between each other in marine ecosystems. Several methods for controlling red tides using mass-cultured algicidal bacteria have been developed. However, to evaluate the efficiency of these methods in natural environments, intensive field testing is required.









![Ingestion rates (IR; cells alga-1 h-1) of red tide dinoflagellate (A) and raphidophyte (B) on bacteria as a function of the initial prey concentration. IR values and regression curves were obtained from Seong et al. (2006). Pm, Prorocentrum minimum; Cp, Cochlodinium polykrikoides; Hr, Heterocapsa rotundata; Ht, Heterocapsa triquetra; Co, Chattonella ovate; Ha, Heterosigma akashiwo. Equations: IR = 21.9 [x/(23.9 × 106+ x)], r2 = 0.668 for Pm; IR = 17.4 [x/(26.3 × 106 + x)], r2 = 0.864 for Cp; IR = 6.0 [x/(3.2 × 106 + x)], r2 = 0.743 for Ht; IR = 11.2 [x/(9.4 × 106 + x)], r2 = 0.709 for Hr; IR = 24.5 [x/(7.2 × 106 + x)], r2 = 0.703 for Co; IR = 11.7 [x/(4.3 × 106 + x)], r2 = 0.771 for Ha.](http://oak.go.kr/repository/journal/12776/JORHBK_2013_v28n4_297_f001.jpg)