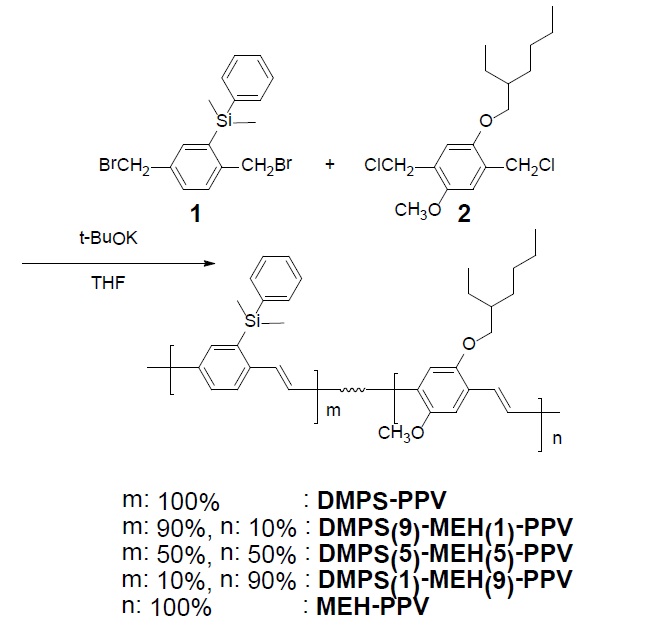
New random copolymers of phenylsilyl- and alkoxy-substituted phenylenevinylene, DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV and DMPS(1)-MEH(9)-PPV, have been synthesized by the Gilch dehydrohalogenation route, and the light-emitting properties of these polymers have been studied. The synthesized polymers were completely soluble in common organic solvents, and exhibited good thermal stability, almost up to 380℃. They showed UV-visible absorbance and photoluminescence (PL) in the ranges of 422-510 and 513-590 nm, respectively, according to their feed ratios. Electroluminescent devices were fabricated with these polymers as emitting layers, and ITO and Al as anode and cathode, respectively. DMPS(1)-MEH(9)-PPV, DMPS(5)-MEH(5)-PPV and DMPS(9)-MEH(1)-PPV exhibited EL emission maxima at 575 nm, 565 nm, and 541 nm, respectively.
Light-emitting polymers have been extensively investigated in recent years, since Burroughes et al. first reported a green light-emitting diode (LED), using poly(
There has been increasing interest in the silyl substituted PPV derivatives as luminescent polymers, since the first siliconcontaining derivative, poly[2-(3-
In this paper, we report the synthesis of random copolymers composed of phenylsilyl- and alkoxy-sbustituted phenylenevinylene, DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV and DMPS(1)-MEH(5)-PPV, via the Gilch dehydrohalogenation method. Depending on the feed ratio of two monomers, emissions and other properties of copolymers were systematically controlled. Single layer EL devices have been fabricated using these polymers as the emissive layers. All the synthetic routes and polymer structures are shown in Scheme 1.
Materials. 2-Bromo-
Instrumentation. 1H NMR and 13C NMR spectra were recorded with the use of Bruker AVANCE 300 and 400 spectrometers, and chemical shifts were reported in ppm units with tetramethylsilane as an internal standard. Chloroform (CDCl3) was mainly used as the solvent for recording NMR spectra. Melting points (mp) were determined, using an Electrothermal model 1307 digital analyzer. Elemental analyses were performed by the Analytical Laboratory of Korea Advanced Institute of Science and Technology, using an Elemental Analyzer (EA1110-FISONS). The FT-IR spectra were obtained by a Bruker EQUINOX 55 spectrometer, by dispersing samples in KBr. Differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) were performed under nitrogen at a heating rate of 10 ℃/min, with a DuPont 9900 analyzer. Gel permeation chromatography (GPC) analysis was conducted on a Waters GPC-150C model system, using polystyrene as standard, and THF as eluent. UV-vis spectra were measured on a Shimadzu UV-3100S spectrometer, and PL spectra of the polymers were obtained using a Spex Fluorolog-3 (Model FL3-11) spectrofluorometer. Absorption and PL spectra were measured on polymer films spin-coated onto quartz substrates. The film thicknesses of the polymers were found by Tencor Alpha-Step 500 surface profiler.
2-Dimethylphenylsilyl-1,4-bis(bromomethyl)benzene (1)
1,4-Bis(chloromethyl)-5-(2-ethylhexyloxy)-2-methoxybenzene (2). The monomer 2 was prepared by reacting 30.0 g (130 mmol) of compound 4-(2-ethylhexyloxy)-1-methoxybenzene with excess hydrochloric acid and formaldehyde in 1,4-dioxane. The reaction solution was heated at 90℃ for 72 hours. The resulting mixture was extracted with ethyl acetate and water. The crude solid was dissolved in a small amount of hexane, and then excess methanol was added. After filtration and vacuum drying, white solid was obtained. The product yield was 30.0 g (71 %); mp 67-68℃. 1H-NMR (CDCl3, ppm) δ 6.7 (d, 2H), 4.4 (s, 4H), 3.7 (d, 2H), 3.6 (s, 3H), 1.7-1.5 (m, 9H), 0.8 (m, 6H). Anal. Cacld for C17H26O2Cl2: C, 61.26; H, 7.88. Found: C, 61.89; H, 7.67.
Poly[2-dimethylphenylsilyl-1,4-phenylenevinylene] (DMPS-PPV). The monomer 1 (0.5 g, 1.26 mmol) was dissolved in 30 mL of THF, and then, the solution was cooled to 0℃. The initiator, 3.78 mL (3.78 mmol) of potassium
Poly[2-dimethylphenylsilyl-1,4-phenylenevinylene-co-2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene]s (DMPS(9)-MEH(1)-PPV). The monomer 1 (0.40 g, 1.01 mmol) and monomer 2 (25 mg, 0.11 mmol) were dissolved in 30 mL of anhydrous THF, and the solution was cooled to 0℃ with an ice bath. The initiator, 3.36 mL (3.36 mmol) of potassium
DMPS(5)-MEH(5)-PPV. The monomer 1 (0.29 g, 0.7 mmol) and monomer 2 (0.15 g, 0.7 mmol) were dissolved in 30 mL of anhydrous THF, and the solution was cooled to 0℃ with an ice bath. The initiator, 4.20 mL (4.20 mmol) of potassium
DMPS(1)-MEH(9)-PPV. The monomer 1 (0.08 g, 0.2 mmol) and monomer 2 (0.38 g, 1.8 mmol) were dissolved in 30 mL of anhydrous THF, and the solution was cooled to 0℃ with an ice bath. The initiator 6.00 mL (6.00 mmol) of potassium
Poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] (MEH-PPV). The well-known MEH-PPV was synthesized by the method of dehydrohalogenation route (the Gilch procedure) using the monomer 2 [5]. The final polymer was soluble in common organic solvents.
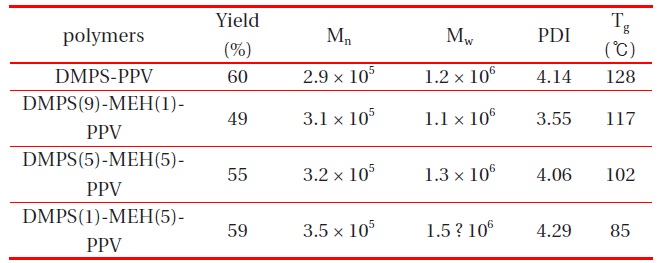
All random copolymers were completely soluble in common organic solvents, such as chloroform, chlorobenzene, THF, 1,2-dichloroethane, and toluene. Huang et al. reported that silylsubstituted PPVs have few structural defects in their polymer chains, and that the silyl groups contribute to the satisfactory solubility of these polymers, because of the larger atomic size of Si compared to C and O, as well as the longer bond length of Si-C compared to C-C and C-O [12]. The molecular weights of the polymers were measured by gel permeation chromatography, using THF as eluent, and polystyrene as standard. The number average molecular weights, Mn, of the DMPS-PPV and copolymers (DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV and DMPS(1)-MEH(9)-PPV) were in the range of 2.9 × 105 and 3.5 × 105, with a polydispersity index (PDI) of 3.55-4.29, respectively. The polymerization results of all synthesized polymers are summarized in Table 1.
In the FT-IR spectra (not shown here), all polymers showed weak but clear absorption peaks at about 960 cm-1, corresponding to the appearance of the out-of-plane bending mode of the
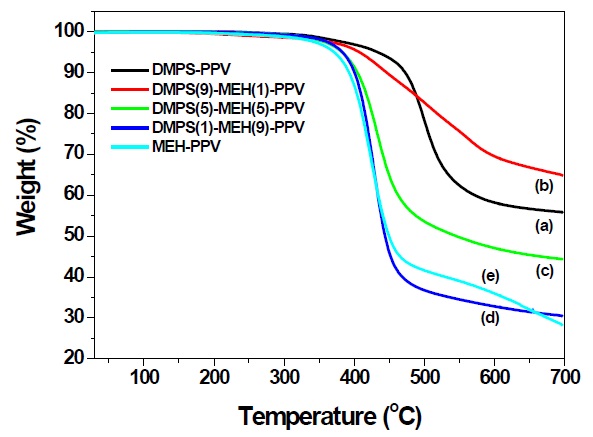
The thermal properties of the synthesized polymers were evaluated by means of thermogravimetric analysis (TGA) under nitrogen atmosphere. TGA thermograms of the DMPS-PPV and random copolymers from the Gilch route showed less than 5% weight loss on heating to about 380℃. DMPS-PPV exhibits excellent thermal stability. The 5% weight loss temperature for DMPSPPV was 419℃. In the case of DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV and DMPS(1)-MEH(9)-PPV copolymers, 5% weight loss temperatures were found at 407, 382, and 383℃, respectively, as shown in Fig. 1. These 5% weight loss temperatures are higher than the 371℃ of well known MEH-PPV.
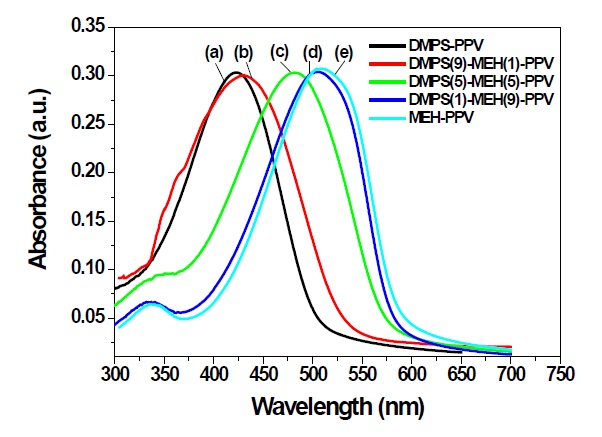
Figure 2 shows the UV absorption spectra of DMPS-PPV, DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV, DMPS(1)-MEH(9)-PPV and MEH-PPV in a thin film onto a quartz plate. The absorption maximum of well known polymer MEH-PPV is found at about 509 nm. But the DMPS(1)-MEH(9)-PPV showed a 502 nm absorption maximum, due to the 10% of phenylsilyl substituted phenylene unit in the copolymer system. The phenylsilyl substituent has little electron-donating property, compared to that of the alkoxy substituent of 2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylene unit. The maximum absorption wavelengths of DMPS(5)-MEH(5)-PPV and DMPS(9)-MEH(1)-PPV appeared at 480 and 430 nm, respectively. The absorption maximum bands of DMPS(5)-MEH(5)-PPV and DMPS(9)-MEH(1)-PPV were more blue-shifted, compared to those of DMPS(1)-MEH(9)-PPV. This means that the π-electron delocalization
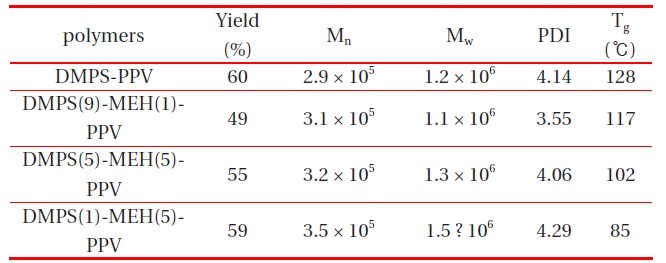
Polymerization results of DMPS-PPV, DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV and DMPS(1)-MEH(5)-PPV.
of the polymer main chain was interrupted by the phenylsilyl substituted phenylene unit, yielding a reduction of the π-conjugation length. Consequently, the π-electron delocalizations of the polymer main chains were systematically controlled by the feed ratios of the phenylsilyl substituted phenylene unit.
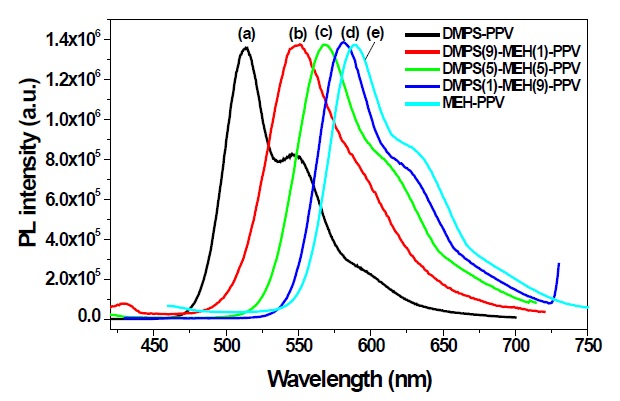
The PL spectra showed drastic changes of emission color through the copolymerization. The PL spectra of the DMPSPPV, DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV, DMPS(1)-MEH(9)-PPV and well known MEH-PPV thin films coated on quartz plates are shown in Fig. 3. These PL spectra were recorded with an excitation wavelength corresponding to the absorption maximum wavelength of the polymer. The PL spectrum of MEH-PPV showed that the emission maximum is about 589 nm. The emission of DMPS(1)-MEH(9)-PPV was blue-shifted about 10 nm because of 10% of phenylsilyl substituted phenylene unit in the copolymer system. As a result of the decrease of the alkoxy substituted phenylene unit in DMPS(1)-MEH(9)-PPV compare with that of MEH-PPV, the electron density of the conjugated main chain in DMPS(1)-MEH(9)-PPV slightly diminished. Consequently, DMPS(1)-MEH(9)-PPV, whose emission is slightly blue-shifted compared with that of MEH-PPV, has a larger band gap than does MEH-PPV.
For the case of DMPS(5)-MEH(5)-PPV and DMPS(9)-MEH(1)-PPV, the emission peaks showed at around 567 and 549 nm, respectively, and were more blue-shifted, compared to that of DMPS(1)-MEH(9)-PPV. These results were caused by the silicon atom of phenylsiylyl substituent, which has a slight electron-donating effect, and the greater steric hindrance of the bulky phenylsilyl group, compared to the alkoxy substituent. These factors lead to a breaking of the coplanarity within the copolymer main chain. Therefore, by increasing the feed ratios of phenylsilyl substituent, the band gap of copolymer is enlarged, and blue-shifted emissions are observed, compared with MEH-PPV.
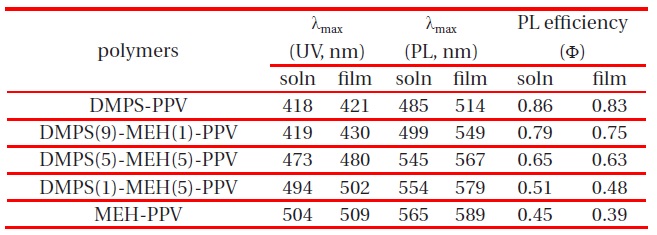
Interestingly, DMPS-PPV is highly fluorescent, in both solution and film [11]. The PL quantum efficiency of synthesized copolymers in chloroform solution was determined twice more, using a dilute quinine sulfate as a standard (ca. 1 × 10-5 M solution in 0.10 M H2SO4) [15]. The measured values were then averaged. The PL efficiency of quinine sulfate in 0.1 M H2SO4 solution was taken to be 0.546 at 365 nm excitation, and the refractive indices of the dilute polymer solutions in chloroform and 0.10 M H2SO4 were taken to be 1.446 and 1.333, respectively. Using these values, the quantum yields of DMPS-PPV, DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV, and DMPS(1)-MEH(9)-PPV were determined as Φsol = 0.86, 0.79, 0.65, and 0.51, which are much higher than those of other PPVs containing alkoxy or alkyl side chains. In considering the utility of these copolymers for applications in thin film devices, the film PL quantum efficiency is more meaningful than the solution value. The film PL quantum efficiencies were measured using an optically dense configuration, and diphenyl anthracene (dispersed in a PMMA film at a concentration of less than 10-3 M, assuming a PL efficiency of 0.83) as the standard [16]. DMPS-PPV, DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV, and DMPS(1)-MEH(9)-PPV showed very high film PL quantum efficiencies of Φfilm = 0.83, 0.75, 0.63, and 0.48, respectively. These values are still higher than those of other PPV derivatives. Chain packing and conjugation are more effectively interrupted in the phenylsilyl substituted phenylene unit, which results in the formation of amorphous polymer thin films for an increased luminescence on increasing the feed ratios of phenylsilyl substituent in copolymers. The absorption and photoluminescence results for DMPS-PPV, DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV, DMPS(1)-MEH(9)-PPV and MEH-PPV in solution and film states are summarized in Table 2.
A single-layer light-emitting diode was fabricated, by using ITO as the anode, and Al as the cathode. DMPS-PPV, DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV, DMPS(1)-MEH(9)-PPV and MEH-PPV polymers were deposited onto indium-tin oxidecovered glass substrates, by spin-casting the soluble polymers in chlorobenzene. The spin-casting technique yielded uniform films, with thicknesses of about 80-90 nm. The Al electrode (cathode) was then evaporated onto this
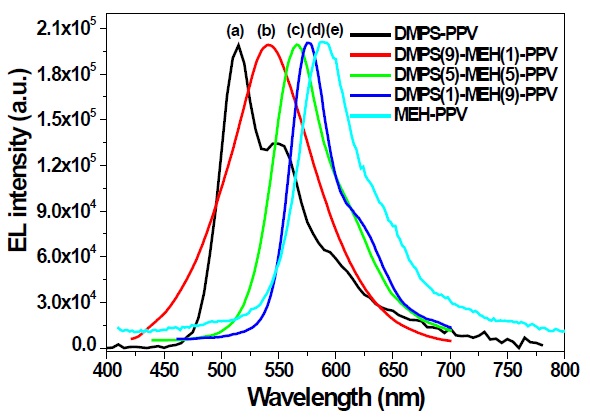
The MEH-PPV polymer film showed maximum EL emission at 590 nm. On increasing the feed ratio of the phenylsilyl substituted phenylene unit, the EL emissions are gradually blueshifted.
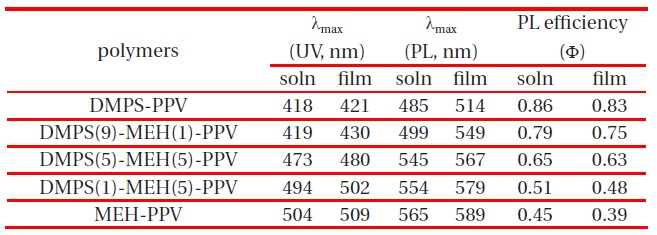
UV-vis spectra, PL spectra and quantum yield in solution and film of DMPS-PPV, DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV, DMPS(1)-MEH(5)-PPV and MEH-PPV.
Therefore, DMPS(1)-MEH(9)-PPV, DMPS(5)-MEH(5)-PPV and DMPS(9)-MEH(1)-PPV exhibited EL emission maxima at 575 nm, 565 nm, and 541 nm, respectively. DMPS-PPV exhibits an EL emissive band at 515 nm. All these EL emission results mean that the π-conjugation length can be controlled by the feed ratio of phenylsilyl substituent in the copolymer system. We propose that the copolymers of phenylsilyl- and alkoxy-substituted phenylenevinylene could be good candidates for light-emitting materials for OLED applications. Further device optimizations are in progress, by replacing the cathode, or the insertion of a hole injection layer, to increase the EL efficiency of the synthesized copolymers. Detailed device works and other electrical data will be published elsewhere.
We have reported the synthesis of novel random copolymers of phenylsilyl- and alkoxy-substituted phenylenevinylene, DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV and DMPS(1)-MEH(9)-PPV, using the Gilch dehydrogalogenation route. The π-conjugation lengths of synthesized polymers were regulated by the feed ratio of phenylsilyl- and alkoxy-substituted phenylene monomers. The synthesized polymers were completely soluble in common organic solvents. They could be spin-cast onto various substrates, to give highly transparent homogeneous thin film without heat treatment. All polymers showed excellent thermal stability up to 380℃ with less than 5% weight loss. In the UVvisible and PL spectra of copolymers, the maximum absorption and emission were controlled by the feed ratio of phenylsilyland alkoxy-substituted phenylene monomers. Consequently, DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV and DMPS(1)-MEH(9)-PPV showed PL emission at 549, 567, and 579 nm, respectively. The phenylsilyl substituted copolymers exhibited very high solution and film PL efficiencies, compared to other alkoxy and alkyl side chains containing PPV derivatives. From the single-layer light-emitting diodes of ITO/copolymer/Al, DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV and DMPS(1)-MEH(9)-PPV gave the highest peaks in the EL emissive band, at 541, 565, and 575 nm, respectively. All characteristics of phenylsilyl- and alkoxy-substituted phenylenevinylene, DMPS(9)-MEH(1)-PPV, DMPS(5)-MEH(5)-PPV and DMPS(1)-MEH(9)-PPV, such as good thermal stability and luminance properties, make them potential candidates for application in polymer LEDs.