Studies were carried out on the speciation of heavy metals (Zn, Cu, Mn, Fe, Ni, Pb, Cd, and Cr) during rotary drum composting of water hyacinth (Eichhornia crassipes) for a period of 20 days. Five different proportions of cattle manure, water hyacinth and sawdust were prepared for composting. This study concluded that, rotary drum was very efficient for the degradation of organic matter as well as for the reduction of mobility and bioavailability of heavy metals during water hyacinth composting. The results from the sequential extraction procedure of heavy metals shows that rotary drum composting changed the distribution of five fractions of Zn, Cu, Mn, Fe, Ni, Pb, Cd, and Cr. The highest reduction in the bioavailability factors of Pb and Cd was observed during the process. The total concentration of Cu, Cr, and Cd was very low compared to the other metals (Zn, Mn, Fe, Ni, and Pb); however, the percentage of exchangeable and carbonate fractions of these metals was similar to other metals. These results confirmed that the bioavailability of metals does not depend on the total concentration of metals. From this study, it can be concluded that the addition of an appropriate proportion of cattle manure significantly reduced the mobile and easily available fractions (exchangeable and carbonate fractions) during water hyacinth composting in rotary drum.
A well-organized and promising technique in decentralized composting is the rotary drum composter, which provides agitation, aeration and mixing of the compost, to produce a consistent and homogeneous end product without any odor- or leachate- related problems [1]. Warm and moist environments with ample amounts of oxygen and organic material available within the rotary drum allow aerobic microbes to flourish and decompose waste more rapidly, resulting in a significantly reduced composting time of 2?3 weeks. Rotary drum composter has been used to compost diverse organic wastes, such as cattle manure, swine manure, municipal biosolids, brewery sludge, chicken litter, animal mortalities, olive mill waste, and food residuals [2-4].
Water hyacinth (
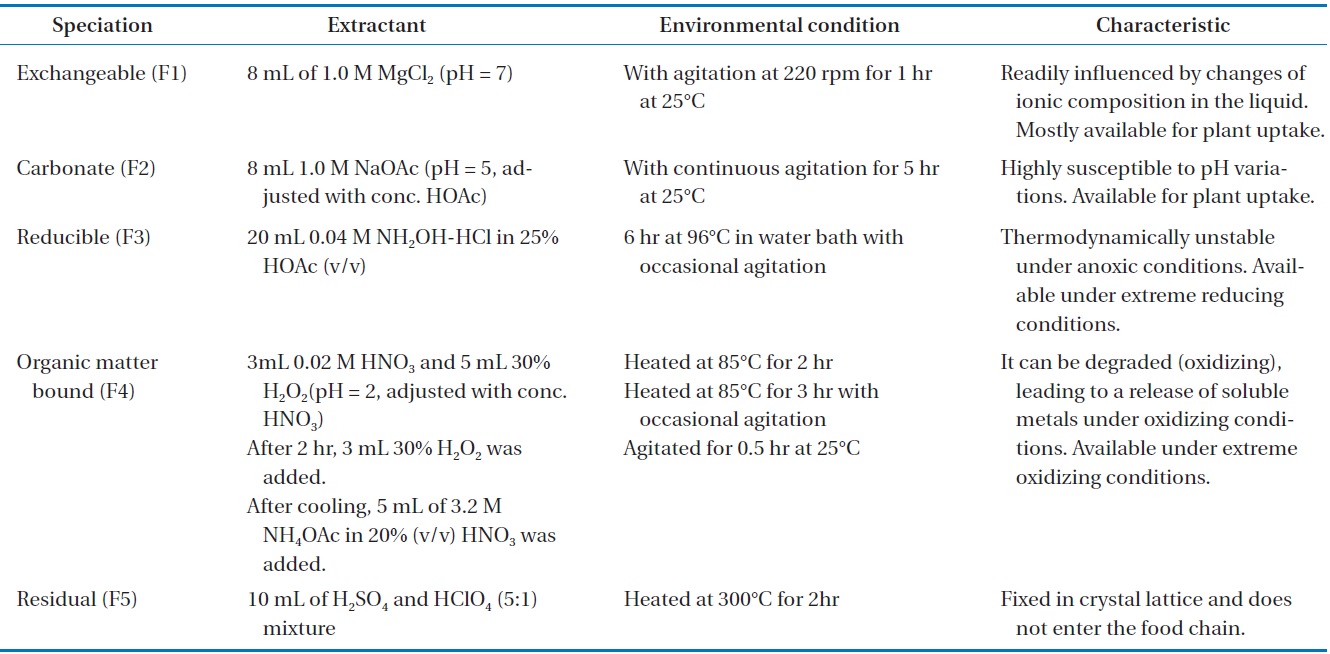
However, the presence of non-biodegradable materials and the high level of toxic heavy metals in the compost frequently hinder agricultural land application. The uptake of heavy metals by plants and the subsequent accumulation along the food chain is a potential threat to animals and human health [8,9]. The total heavy metal concentration does not provide valuable information regarding the risk of bioavailability, which depends on their chemical forms; however, it can be used as an overall pollution indicator [10,11]. The evaluation of the chemical speciation of heavy metals in composts permits access to their bioavailability and suitability for land application [8]. The sequential chemical extraction procedure could provide an understanding of the heavy metal speciation with different natures and also allow for the prediction of metal mobility, bioavailability and leaching rates [12]. The mobility and bioavailability of metals are decreased approximately in the order of extraction sequence [13]. According to the procedure of Tessier et al. [14], heavy metals are associated with five fractions: exchangeable fraction (F1), carbonate fraction or acid extractable (F2), reducible fraction (F3), oxidizable fraction or fraction bound to organic matter (F4), and residual fraction (F5). The F1 and F2 fractions of the total metals are considered to be the most plant-available form, F3 and F4 fractions are available for plants in extreme environmental conditions, but residual fraction is never obtainable for plants [11]. Although various studies have been conducted on the speciation of heavy metals regarding traditional composting techniques, such as windrows/piles types of composting for various kinds of wastes [9-11,15-17], there is information available on the speciation of heavy metals during rotary drum composting of water hyacinth.
Therefore, the objectives of the current study were to assess the effect of rotary drum on the speciation of heavy metals (Zn, Cu, Mn, Fe, Pb, Ni, Cd, and Cr), and to investigate the effect of physicochemical parameters on metal speciation during composting of water hyacinth.
Water hyacinth, cattle (cow) manure and sawdust were used for the preparation of different waste mixtures. Water hyacinth was collected from the Amingaon industrial area near the Indian Institute of Technology Guwahati campus. Cattle manure was obtained from a dairy farm near the campus. Sawdust was purchased from a nearby saw mill. Prior to composting, the maximum particle size in the mixed waste was restricted to 1 cm in order to provide better aeration and moisture control. The composition and initial characterizations of waste materials (water hyacinth, cattle manure and sawdust) are given elsewhere Singh and Kalamdhad [18].
2.2. Rotary Drum Composter Design
The design of the pilot-scale rotary drum composter was used similar to that of Singh and Kalamdhad [18]. A pilot-scale rotary drum composter with a capacity of 550 L was operated using a batch-mode operation. The main unit of the composter,i.e., a drum of 1.22 m in length and 0.76 m in diameter, is fabricated by a 4-mm thick metal sheet. The inner side of the drum is covered with an anti-corrosive coating. The drum is mounted on four rubber rollers attached to a metal stand and is rotated manually with its handle. The 40 × 40 mm angles are welded longitudinally inside the drum for proper mixing, agitation and aeration of the wastes during rotation. The ability of the pilot-scale rotary drum composter was decided by keeping in view of the potential of a single person who can handle around 150 kg of wastes by manual rotation. The composting period of 20 days was decided for proper degradation and stabilization based on the performance of earlier studies, which have been performed by in-vessel composting reactors [1]. Manual turning was completed after every 24 hr through one complete rotation of the rotary drum in order to ensure that the material on the top portion moved to the central portion, where it was subjected to higher temperature. Subsequently, the aerobic condition was maintained by opening the top half side doors of the two circular faces.
The samples were collected through grab sampling from different locations, mainly from the midspan and end terminals of the pilot-scale rotary drum composter, utilizing a compost sampler to minimize the disturbance of the adjacent materials. All the grab samples were thoroughly mixed together to make a homogenized sample. Triplicates homogenized samples were collected on the 0, 4th, 8th, 12th, 16th, and 20th day after manually turning by hand. Thesamples were dried at 105℃ in the oven for 24 hr and then, the moisture content was calculated. The dried samples were ground in order to pass through 0.2 mm sieves and were then stored for further analysis.
Temperature was monitored using a digital thermometer throughout the composting period. Each ground sample was analyzed for the following parameters: pH (1:10 w/v waste, water extract) and total organic carbon (TOC) [19]. Total metals (Zn, Cu, Mn, Fe, Pb, Ni, Cd, and Cr) were determined by atomic absorption spectrometer (AAS, VarianSpectra AA 55B; Varian Inc., Palo Alto, CA, USA) after the digestion of a 0.2 g sample with 10 mL of H2SO4 and HClO4 (5:1) mixture in the block digestion system (Pelican Equipments, Chennai, India) for 2 hr at 300℃.
2.4. Methodology of Sequential Extraction
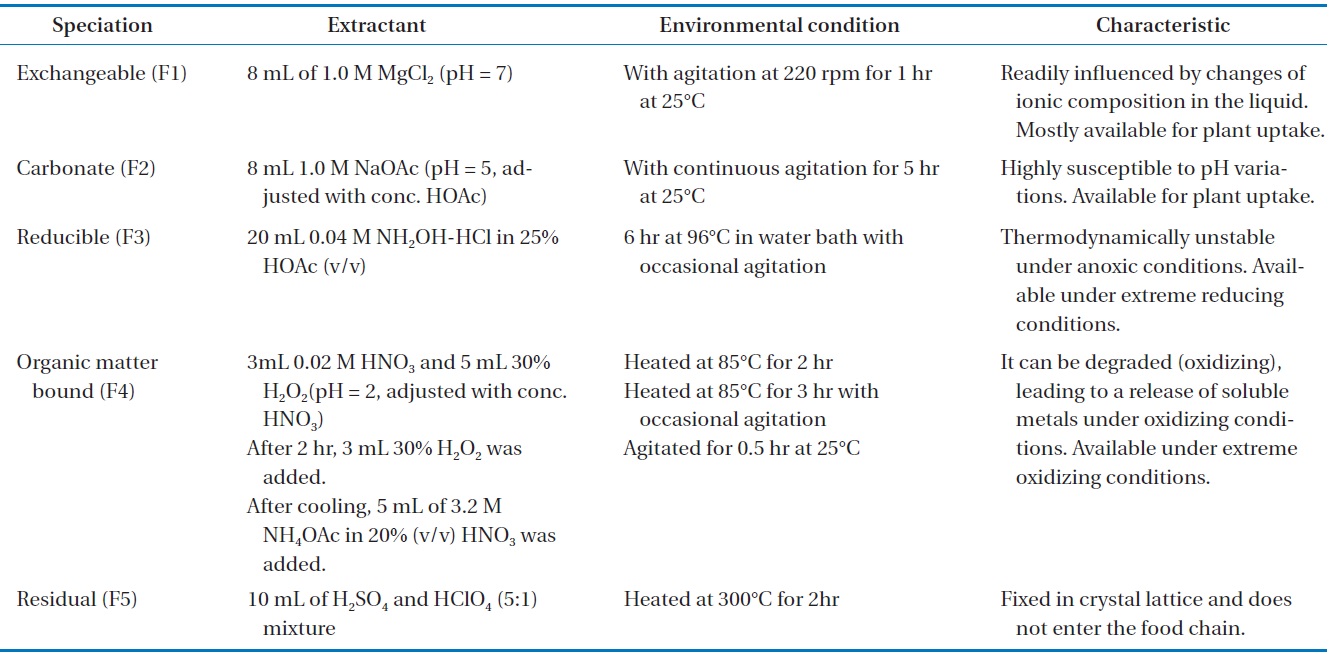
The conventional method for heavy metal speciation was designed and developed by Tessier et al. [14]. It was divided into the following five species: exchangeable (F1), carbonate (F2), reducible (F3), oxidizable (F4), and residual (F5) fraction. The detail methodology of sequential extraction was provided in Table 1. AAS was used for the analysis of metals in the extracted samples from the different steps. The sum of the total concentration of F1, F2, F3, and F4 for one heavy metal represents its total concentration of mobile fractions (FA) in mg/kg (dry matter). Furthermore, FT is defined as the sum of the total concentration of F1, F2, F3, F4, and F5 fractions of the heavy metal in mg/kg (dry matter); the bioavailability factors (BF) of one heavy metal is defined as the ratio of FA to FT.
BF = FA / FT
All physicochemical analyses were repeated three times. All repeated measures treated with the analysis of variance (ANOVA) were made using the statistical software. The objective of the statistical analysis was to determine any significant difference among the parameters; different forms of metals were analyzed for different trials.
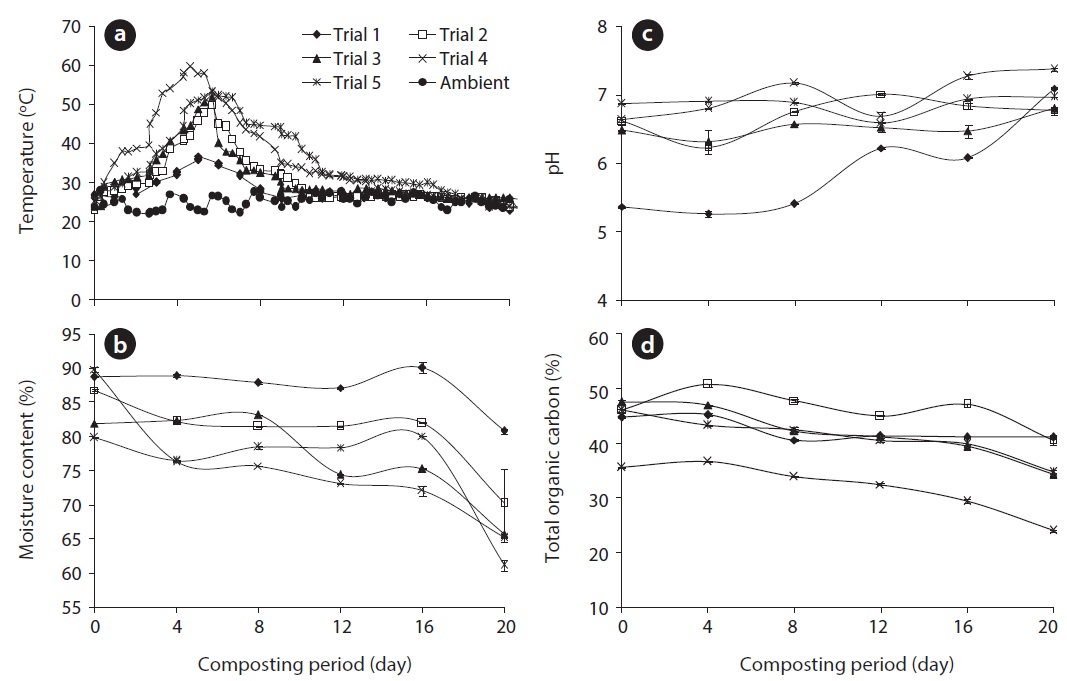
The progression of temperature during high rate composting in the rotary drum composter is presented in Fig. 1. The microbial activities began during the initial hours of process, which caused an increase in temperature. Temperature from 52℃ to 60℃ is measured to maintain the greatest thermophilic activity in the composting systems [19]. As shown in Fig. 1, the temperature of the composting trials went through three typical phases (mesophilic, thermophilic, and cooling phases) and ranged from 22℃ to 60℃ during the entire period of composting. The addition of cattle manure may have influencedrature during the composting process in the five different trials. Out of all five trials, trial 4 reached the highest temperature due to the higher addition of cattle manure, representing a rapid establishment of microbial activity during the composting process. These results indicate that cattle manure improved the composting process by easily providing available carbon for microorganisms; however, in trial 5, which contained the highest amount of cattle manure, shows less temperature when compared to trial 4. Although excess cattle manure content may increase the available carbon, there may be inadequate nitrogen, porosity and oxygen in trial 5 thereby reducingrature; furthermore, compost microorganisms may require optimum carbon, nitrogen, and oxygen for their survival.
Extractants, experimental conditions, and properties of various extractable heavy metal forms with sequential extraction
Moisture loss during the composting process in the form of vapor by heat production can be viewed as an index of the decomposition rate [19]. Composting materials require the most favorable moisture content for the survival of organisms. The initial moisture content was 88.8%, 86.9%, 81.9%, 89.9%, and 79.5%, which was reduced to 86.2%, 80.7%, 76.2%, 65.3%, and
78.7% within 20 days of the composting period in trials 1, 2, 3, 4, and 5, respectively (Fig. 1). The highest moisture loss occurred in trial 4 (27.4%), whereas the lowest moisture loss was observed in trial 1 (8.9%), due to high heat generation in trial 4 and low heat generation in trial 1. By analyzing the results of ANOVA, the decrease in moisture content varied significantly between different days (
The pH is the parameter which can greatly affect the composting process. The pH values observed in all trials were within the optimal range for the development of bacteria (6.0?7.5) and fungi (5.5?8.0) [15]. Fig. 1 indicates the variation in pH among five different trials during the composting process. The pH values were increased in all trials; in particular, the maximum pH 7.4 was observed in trial 4 at the end of the composting process. A significant difference in pH was observed in all trials (
TOC content decreased significantly during composting (Fig. 1), which is reliable with the breakdown of the organic matter through microbe respiration in the form of CO2 and even throughout mineralization [21]. Organic matter was decomposed and transformed into stable humic compounds. Humic substances have a capacity to interact with metal ions and also have the ability to buffer pH and to act as a potential source of nutrients for plants [15]. The maximum TOC loss was observed in trial 4 (32.6%), followed by trial 3 (28%), trial 5 (24.5%), trial 2 (10.6%) and trial 1 (8.0%). Higher TOC loss occurred during the composting process as a result of higher temperature during the thermophilic phase of trial 4. A significant variation in TOC was observed in all trials (
3.2. Heavy Metal Speciation during Composting
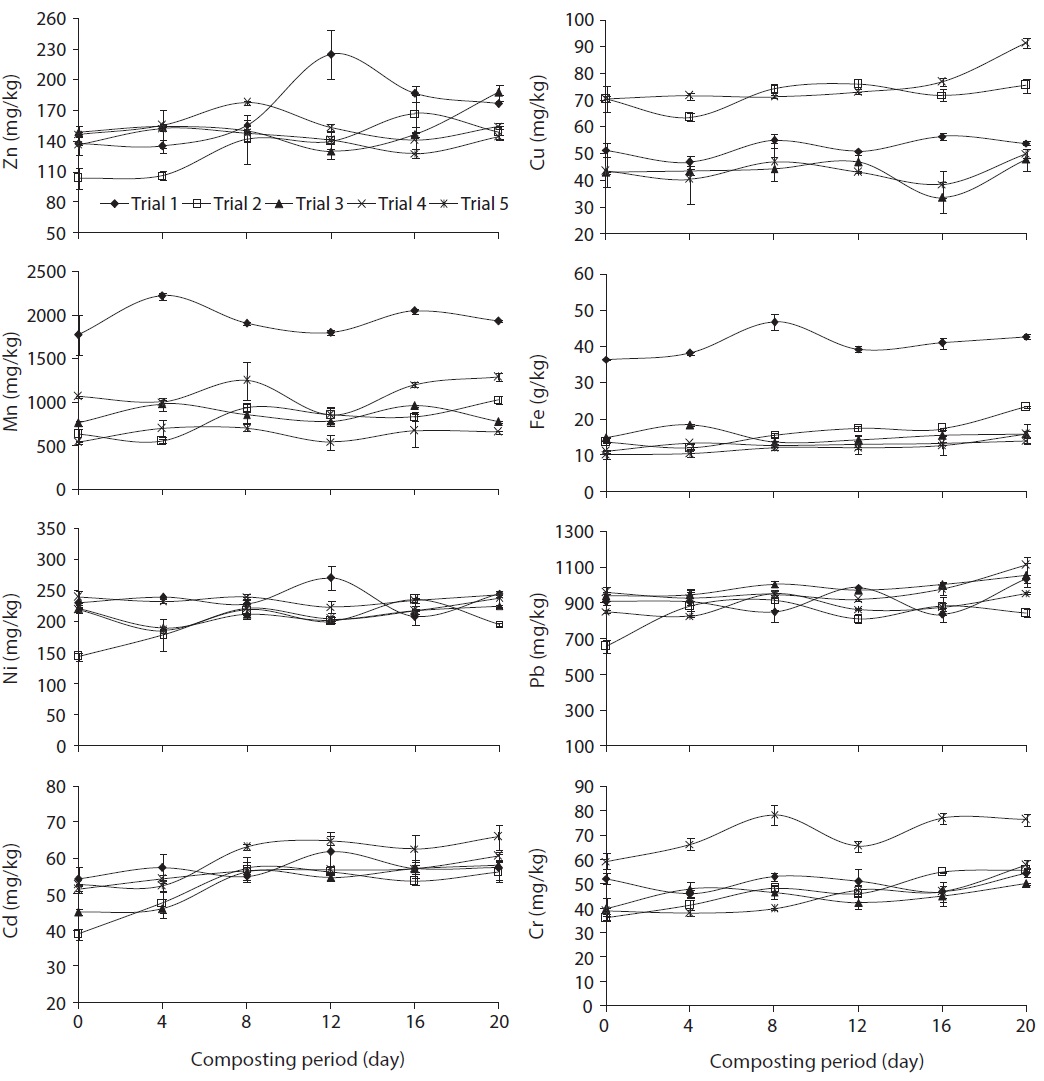
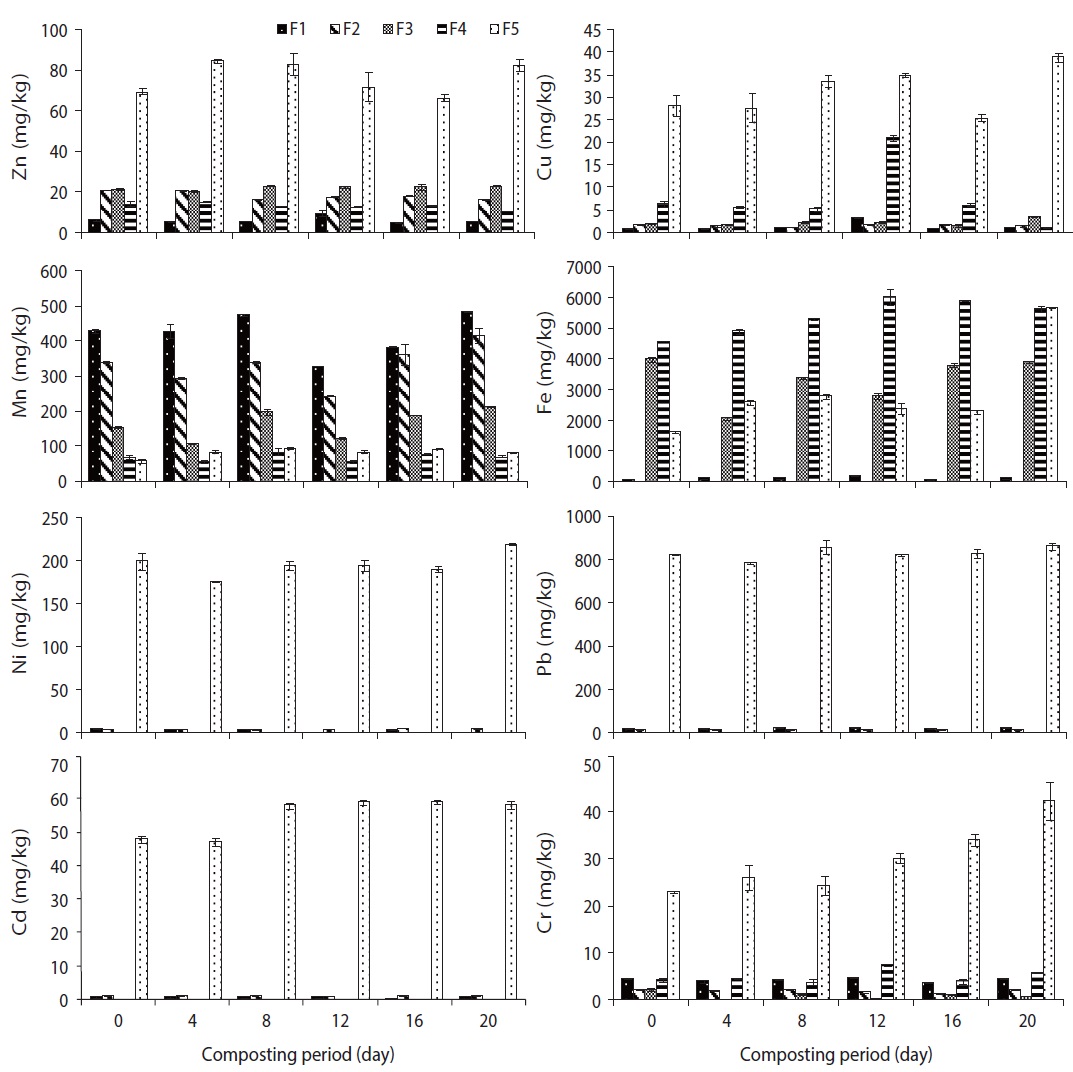
Fig. 2 illustrates the total concentration of metals (Zn, Cu, Mn, Fe, Ni, Pb, Cd, and Cr) in trials 1, 2, 3, 4, and 5 during the composting process. These heavy metals were concentrated during the composting process, which was reported by Liu et al. [22], due to the weight loss in the course of composting following organic matter decomposition, release of CO2 water and mineralization processes. The order of total metal content in the composted water hyacinth was Fe > Pb > Mn > Ni > Zn > Cu > Cr > Cd. Furthermore, the increasing trends of total heavy metals concentration was good compared to the traditional composting methods due to the fast rate of degradation and homogenized compost mass in the rotary drum.
3.2.1. Speciation of Zn, Cu, Mn, and Fe
A few metals, including Cu, Mn, Fe, and Zn, are essential for plant metabolism in trace amounts. If extreme levels of these metals are present in bioavailable form, they may potentially be toxic to plants. In water hyacinth, sawdust, and cattle manure, the order of different fractions of Zn is: F5 (63.6%) > F4 (12.4%) > F1 (10.4%) > F3 (8.1%) > F2 (5.6%); F5 (76.7%) > F1 (9.9%) > F2 (7.2%) > F3 (4.5%) > F4 (1.6%); and F5 (53.3%) > F2 (23.5%) > F3 (9.5%) > F4 (8%) > F1 (5.6%), respectively [23].
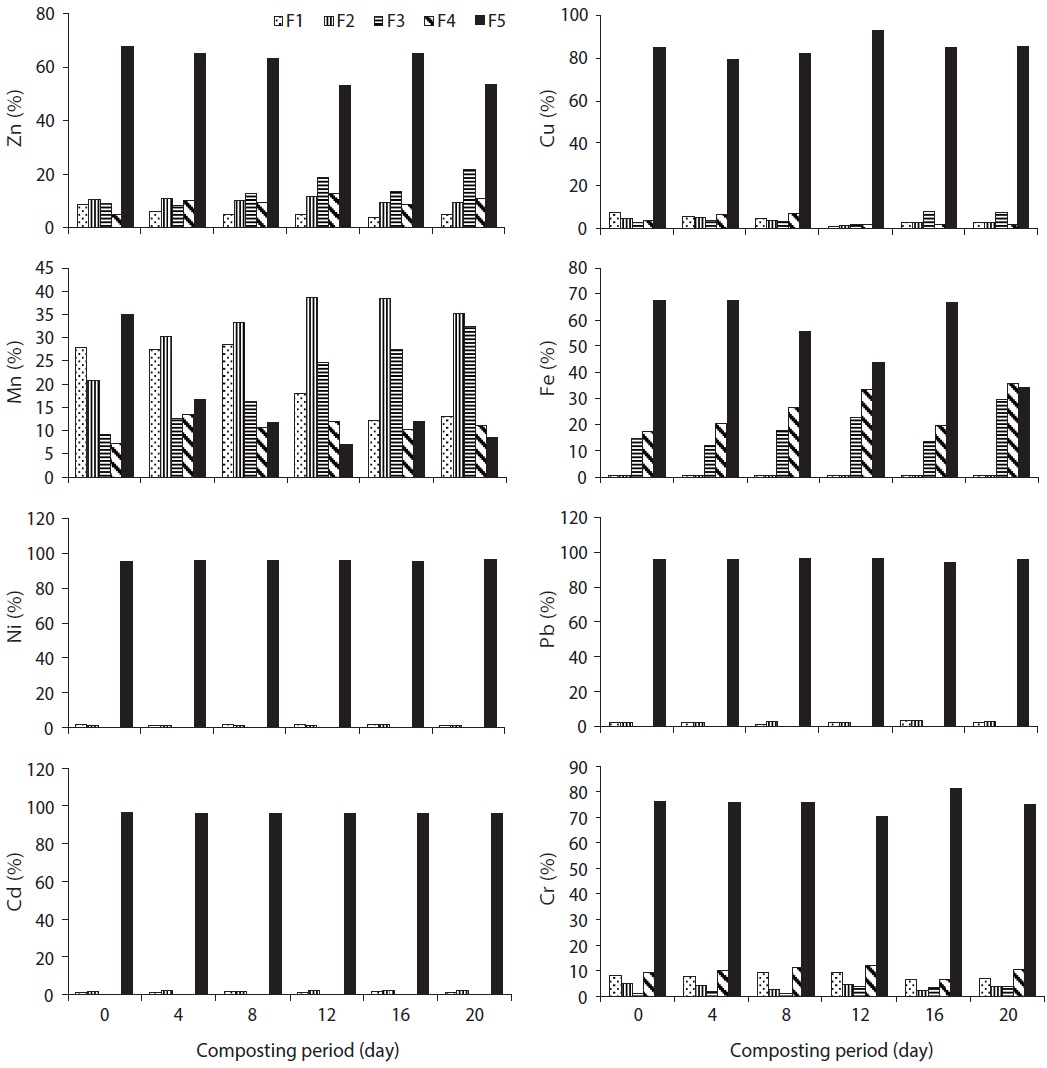
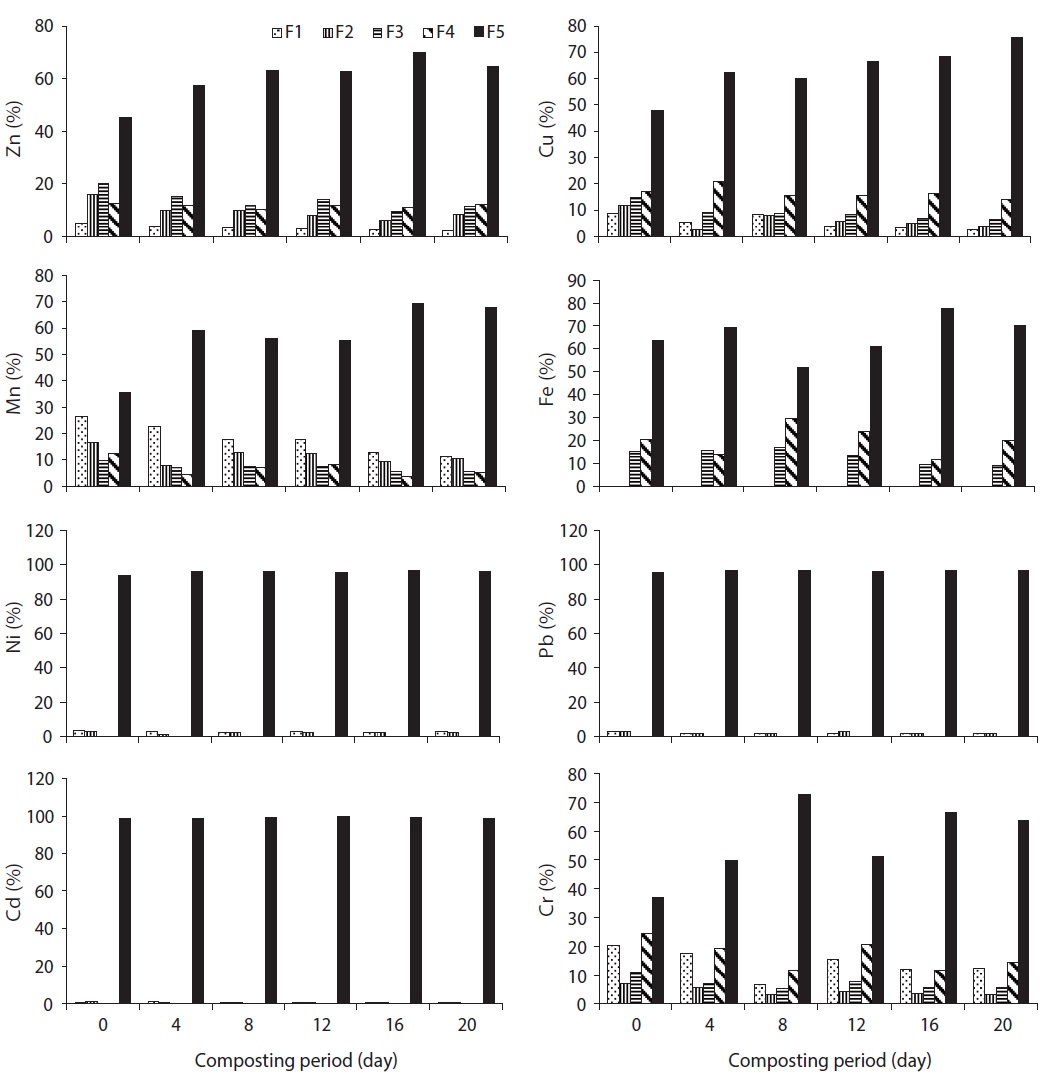
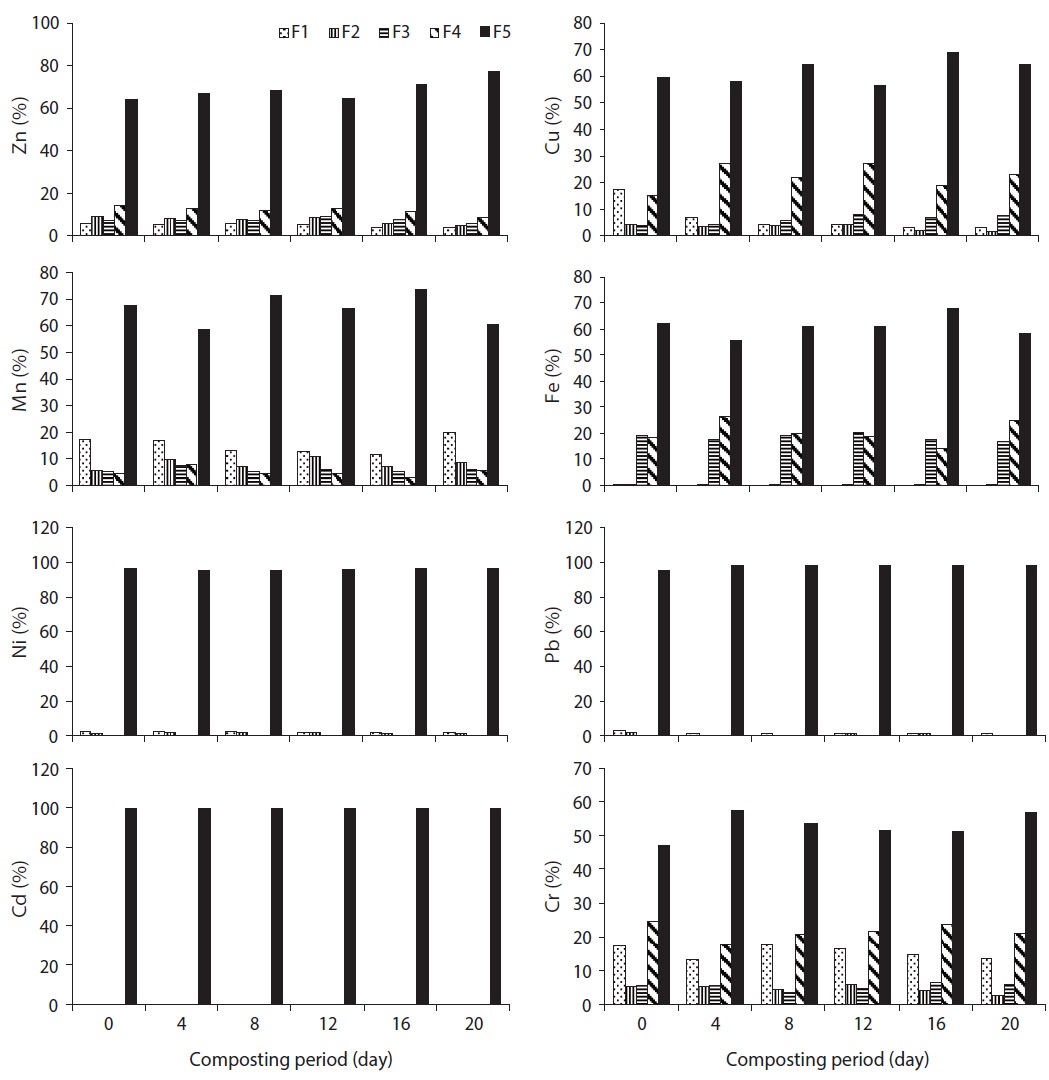
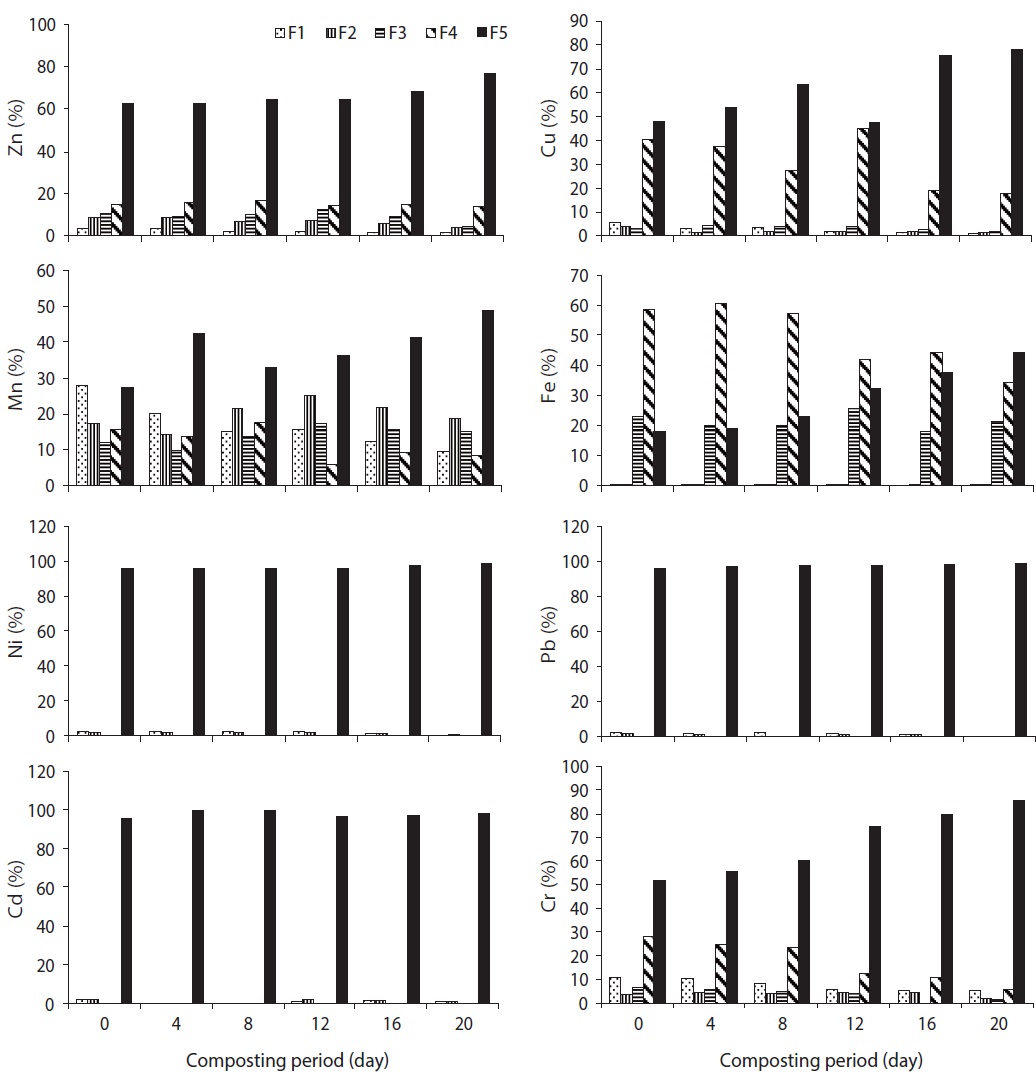
The speciation of Zn in trials 1, 2, 3, 4, and 5 is given in Figs. 3?7, respectively, during the composting process. For Zn, it could be found that the F5 fraction was dominant in all three raw materials. In trial 1, F1, F2, and F5 fractions of Zn were decreased; however, F3 and F4 fractions were increased with regards to the total fraction of Zn during the composting process. In trial 2, F1, F2, F3, and F4 fractions of Zn were decreased; however, the F5 fraction was increased with regards to the total fraction of Zn during the composting process. In trial 3, F2, F3, F4, and F5 fractions of Zn were increased; alternatively, F1 fraction was increased with regards to the total fraction at the end of composting. The stabilization of raw organic matter during composting transformed Zn from F1 fraction to a more stable F4 fraction [24]. In trial 4, F1, F2, F3, and F4 fractions were decreased, whereas F5 fraction was increased based on the percentage of total fraction during the composting process. In trial 5, F1 and F4 fractions were decreased while F2, F3, and F5 fractions were increased with regards to the total fraction during the composting process. The reduction of movable forms might be due to the formation of the Zn complex with humic substances formed at the maturity stage of the compost. Humic substances surround various organic functional groups that can sorb metal ions through ionic force [25].
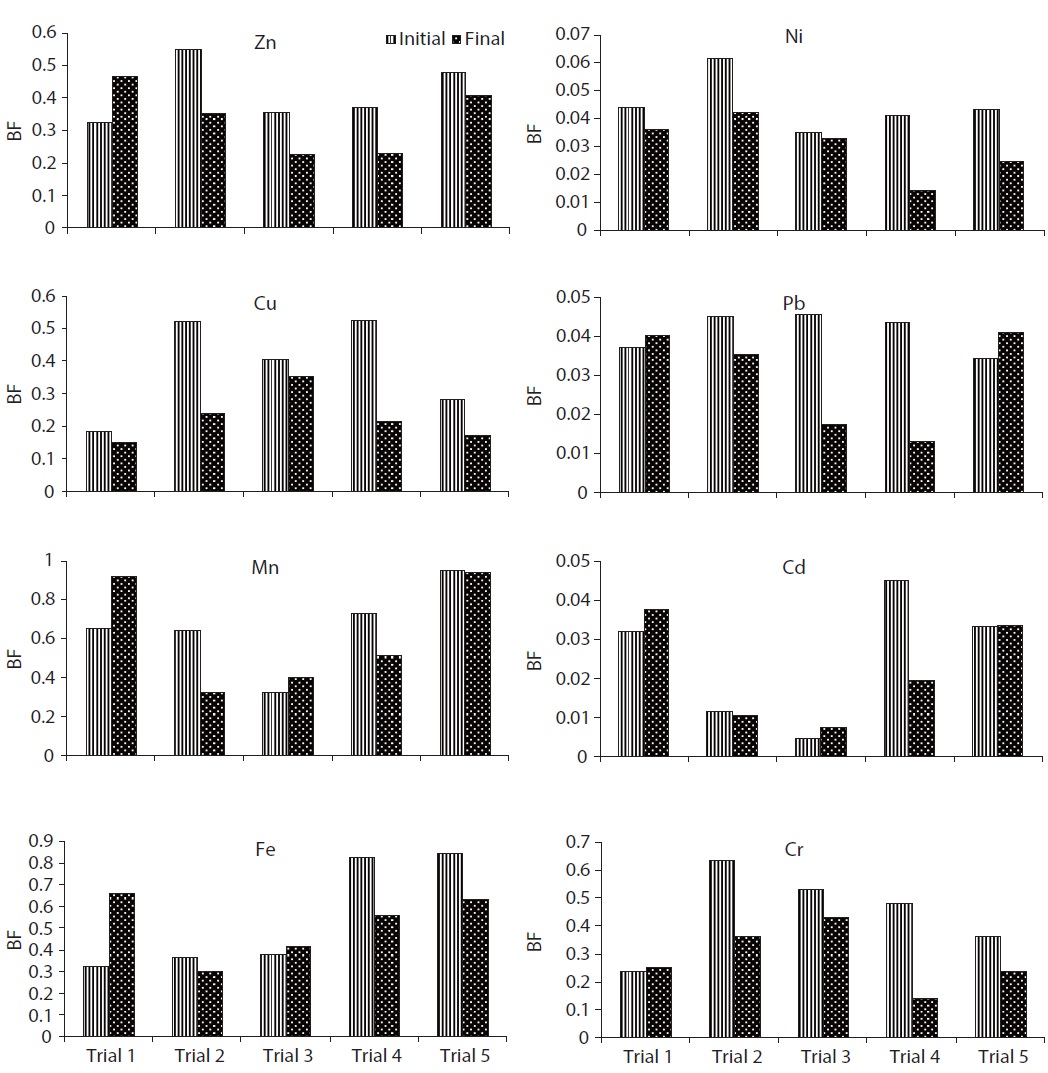
F5 fraction was the predominant fraction of Zn in all trials. In this study, the pH of the final composts ranged from 5.3 to 7.4 (Fig. 1). The pH and transformation of organic matter to humic substances during the composting process might affect the transformation of Zn and consequently, the speciation distribution. The BF of Zn in trials 1 and 3 were increased from 0.32 and 0.25 (initial) to 0.47 and 0.43 (final); however, in trials 2, 4, and 5, it was decreased from 0.59, 0.4, and 0.48 (initial) to 0.25, 0.23, and 0.41 (final), respectively, during the composting period (Fig. 8). The higher reduction in BF of Zn was observed in trial 4 (37.5%), followed by trial 3 (36.5%), trial 2 (35.8%) and trial 5 (14.9%) during the process. The reduction in BF of Zn could be attributed toBF (F1, F2, F3, and F4), which precipitated with hydroxides, carbonates, phosphates, sulfides, and some other anions form complexes with organic ligands. Cation exchange and complexation by organic ligands were suggested to be the main Zn mobility controlling mechanisms [26].
Phytotoxicity could be caused by the high Zn bioavailability factor [27]. The Zn availability was influenced by the total Zn content, pH, organic matter content and the availability of adsorption sites [28]. Significant difference in F1, F2, F3, F4, and F5 fractions of Zn was observed in all trials (
In water hyacinth, sawdust and cattle manure, the order of different fractions of Cu is as follows: F5 (55.6%) > F4 (34%) > F1 (4.7%) > F2 (3.2%) > F3 (2.4%); F5 (59.1%) > F4 (29.9%) > F1 (5.8%) > F3 (3.2%) > F2 (2.1%); and F5 (48.9%) > F4 (38.2%) > F1 (7.0%) > F2 (3.9%) > F3 (2.0%), respectively. The speciation of Cu in trials 1, 2, 3, 4, and 5 is given in Figs. 3?7, respectively, during the composting process. For Cu, it could be found that the F4 and F5 fractions were predominant in all three raw materials [23]. In trial 1, F1 and F2 fractions were decreased although the F3, F4, and F5 fractions were increased with regards to the total fraction of Cu during the composting process. Initially, the F4 fraction of Cu was not present, but at the end of composting, this fraction was increased to 2.2% of the total fraction. In trial 2, F1, F2, F3, and F4 fractions of Cu were decreased; however, the F5 fraction was increased with regards to the total fraction of Cu during the composting process. In trial 3, F1 and F2 fractions of Cu were decreased; however, F3, F4, and F5 fractions were increased from the total fraction of Cu during the composting process.
In trial 4, F1, F2, F3, and F4 fractions of Cu were decreased from 5.2%, 3.6%, 3.1%, and 40.5% to 1.0%, 1.2%, 1.8%, and 17.6% of the total fraction of Cu, whereas the F5 fraction was increased from 47.7% to 78.4% of the total fraction during the composting process. In this trial, all movable fractions of Cu (F1, F2, F3, and F4 fraction) were converted into the F5 fraction at the end of the composting process, which might be due to the formation of the Cu ion complex with two or more organic functional groups, which are primarily carboxylic, carbonyl and phenolic, so that the ion is immobilized in a rigid inner-sphere complex [29]. In trial 5, F2 and F4 fractions of Cu were decreased, whereas F1, F3, and F5 fractions were increased (as the percentage of total fraction) during the composting process (Fig. 4). In this trial, F2 and F4 fractions might have been converted into the F5 fraction during the process. The reduction of all movable fractions (from F1 to F4) of Cu was observed in trial 4 in comparison to other trials; this was due to the maximum organic matter loss, high heat generation and organic mature converted into humic substances.
The BF of Cu was reduced from 0.16, 0.52, 0.40, 0.52, and 0.28 to 0.15, 0.24, 0.35, 0.22, and 0.17 in trials 1, 2, 3, 4, and 5, respectively, during the composting period (Fig. 8). The higher reduction in BF of Cu was observed in trial 4 (58.7%), followed by trial 2 (53.7%), trial 5 (40.0%), trial 3 (11.8%) and trial 1 (6.0%) during the process. The maximum reduction of BF of Cu was observed in trial 4 could be explained as, groups of -OH and ?COOH supplied with cattle manure increased the binding sites and combined with Cu to form insoluble and immobile complexes [30]. Furthermore, the addition of an appropriate proportion of cattle manure enhanced the composting process and consequently improved humic substance formation during the process. Humic substances generally have higher affinity to complex with copper than zinc. This is because water soluble humic substances always have high contents of carboxyl groups, which are ready to complex with Cu [31]. Significant differences in F1, F2, F3, F4, and F5 fractions of Cu were observed in all trials (
In water hyacinth, sawdust and cattle manure, the order of different fractions of Mn is as follows: F5 (40.3%) > F1 (27.6%) > F4 (12.6%) > F3 (11.3%) > F2 (8.2%); F5 (56.5%) > F1 (30.3%) > F2 (6.4%) > F3 (5.1%) > F4 (1.7%); and F1 (43.5%) > F5 (23.5%) > F2 (23.2%) > F3 (7%) > F4 (2.8%), respectively. In water hyacinth and sawdust, F1 and F5 fractions were predominant, but
in cattle manure, the F1 fraction of Mn was predominant [23]. The speciation of Mn in trials 1, 2, 3, 4, and 5 is given in Figs. 3?7, respectively, during the composting process. In trial 1, F1 and F5 fractions of Mn were decreased; however, F2, F3, and F4 fractions were increased from the total fraction of Mn during the composting process. In trial 2, F1, F2, F3, and F4 fractions of Mn were decreased; however, the F5 fraction was increased from the total fraction of Mn during the composting process. In trial 3, F3 and F4 fractions of Mn were decreased; however, F1, F2, and F5 fractions were increased with the total fraction of Mn during the composting process.
In trials 4 and 5, F1 and F4 fractions of Mn were decreased; however, F2, F3, and F5 fractions were increased with regards to the total fraction of Mn during the composting process. These fractions are easily available for plants. The rise in pH can decrease Mn mobility by precipitate formation, thus increasing the number of adsorption sites and decreasing the competition of H+ for adsorption, followed by an increase in metal stability with humic substances [28].
The BF of Mn in trials 1 and 3 was increased from 0.7 and 0.3 (initial) to 0.9 and 0.4 (final), respectively; however, in trials 2, 4, and 5, the BF was decreased from 0.6, 0.7, and 0.94 (initial) to 0.3, 0.5, and 0.93, respectively, during the composting period (Fig. 8). The higher reduction in BF of Mn was observed in trial 2 (50.0%), followed by trial 4 (29.6%) and trial 5 (0.94%) during the process. Significant differences in F1, F2, F3, F4, and F5 fractions of Mn were observed in all trials (
The speciation of Fe in raw materials and the order of different fractions of Fe in water hyacinth, sawdust and cattle manure were as follows: F4 (54.8%) > F3 (30.2%) > F5 (13.9%) > F1 (0.8%) > F2 (0.3%); F5 (47.3%) > F3 (29.5%) > F4(19.3%) > F2 (2.4%) > F1 (1.6%); and F5 (45.8%) > F4 (26%) > F3 (25.2%) > F2 (1.9%) > F1 (1.1%), respectively. In the water hyacinth, F3 and F4 fractions of Fe were predominant [23]. The speciation of Fe in trials 1, 2, 3, 4, and 5 is given in Figs. 3?7, respectively, during the composting process. In trial 1, F1, F2, and F5 fractions of Fe were decreased, but F3 and F4 fractions were increased. In trial 2, F1, F2, F3, and F4 fractions of Fe were decreased although the F5 fraction was increased. In trial 3, F1, F2, F3, and F5 fractions of Fe were decreased; however, the F4 fraction was increased from the total fraction of Fe during the composting process.
In trial 4, F1, F2, F3, and F4 fractions of Fe were decreased;
however, the F5 fraction was increased as the percentage of the total fraction of Fe during the composting process. In trial 5, F1, F2, and F4 fractions of Fe were decreased; however, F3 and F5 fractions were increased from the total fraction of Fe during the composting process. In trials 1, 2, and 3, the F5 fraction was dominant from the initial to the final stage of the composting process due to the addition of less quantity of cattle manure in trials 2 and 3, and nil in trial 1. In trials 4 and 5, Fe was mainly present in the F4 fraction. Due to proper degradation in trials 4 and 5, the F5 fraction of Fe was converted in to the F4 fraction. F1 and F2 fractions of Fe were <2% of the total fractions, despite the fact that the total concentration was very high. Fe was primarily present in the F4 fraction in all trials. The BF of Fe in trials 1 and 3 were increased from 0.32 and 0.38 (initial) to 0.66 and 0.42 (final), respectively; however, in trials 2, 4, and 5, the BF of Fe was decreased from 0.37, 0.82, and 0.84 (initial) to 0.30, 0.56, and 0.63, respectively, during the composting period (Fig. 8). The higher reduction in BF of Fe was observed in trial 4 (32.15%), followed by trial 5 (25.1%) and trial 2 (18.1) during the process. The reduction in BF of Fe could be explained as humic substances formed a complex compound with Fe [23]. Significant differences in F1, F2, F3, F4 and F5 fractions of Fe were observed in all trials (
3.2.2. Speciation of Ni, Pb, Cd, and Cr
The Ni and Cr are required in very less quantity; yet, Pb and Cd are not essential for both plants and animals, even though a very low quantity of Pb and Cd is toxic for plants and humans. The speciation of Ni in raw materials and the order of different fractions of Ni in water hyacinth, sawdust and cattle manure are as follows: F5 (92.8%) > F1 (6.4%) > F2 (0.8%); F5 (98%) > F1 (2.0%); and F5 (97.6%) > F1 (2.4%), respectively [23]. Ni occurred predominantly in the F5 fraction, which agreed with the report of Wang et al. [17]. The speciation of Ni in trials 1, 2, 3, 4, and 5 is given in Figs. 3?7, respectively, during the composting process. After composting, the F5 fraction was still the dominant fraction. In water hyacinth, composting F3 and F4 fractions were not detectable, whereas in sawdust and cattle manure, F2, F3, and F4 fractions were not detectable. In trial 1, F1 and F2 fractions of Ni were decreased; however, the F5 fraction was increased from the total fraction of Ni during the composting process. However, in trials 2 and 4, F1 and F2 fractions of Ni were decreased, whereas
the F5 fraction was increased from the total fraction of Ni during the composting process. The F3 and F4 fractions of Ni were not detected in all trials during the composting period. The maximum reduction of F1 and F2 fractions of Ni were observed in trial 4. In trials 3 and 5, the F1 fraction was reduced, but the F2 and F5 fractions were increased. Through the composting process, the proportion of F1 and F2 fractions of Ni were decreased while the proportion of the F5 fraction of Ni was increased; this was due to F1 and F2 fractions of Ni, which were transformed into the F5 fraction during the composting process [17,32].
Su and Wong [33] reported that the F5 fraction contributes 52% of the total Ni content, followed by the reducible form in sewage sludge. However, in the present study, the F5 fraction contributed about 95% of the total fraction. Fuentes et al. [27] also reported that the maximum amount of Ni was bound with the F5 fraction. A significant increase in the level of Ni with the F5 fraction could be due to the alkaline stabilization process. Both F1 and F2 fractions contributed <10% of the total fraction of Ni in all trials. Approximately 15%?30% in F1 and F2 fractions can cause environmental toxicity during mobility [11]. The BF of Ni was reduced from 0.044, 0.061, 0.034, 0.041, and 0.043 (initial) to 0.036, 0.042, 0.033, 0.014, and 0.025 (final) in trials 1, 2, 3, 4, and 5, respectively (Fig. 8). The higher reduction in BF of Ni was observed in trial 4 (65.0%), followed by trial 5 (42.6%), trial 2 (31.3%), trial 1 (17.1%) and trial 3 (6.2%) during the process. A significant decrease of BF in trial 4 confirmed that the addition of cattle manure in an appropriate proportion could effectively prevent the availability of Ni for plant uptake. Furthermore, the reduction in BF of Ni might be due to the formation of organometallic complex at the maturity of the composting process [29]. Significant differences in F1, F2, and F5 fractions of Ni were observed in all trials (
In water hyacinth, sawdust and cattle manure, the order of different fractions of Pb are as follows: F5 (96.8%) > F1 (2.4%) > F2 (0.8%); F5 (98.7%) > F1 (1.3%) > F2 (0.06%); and F5 (98.4%) > F1 (1.6%), respectively. Pb occurred predominantly in the F5 fraction in all the raw materials. In water hyacinth and sawdust, F3 and F4 fractions were not detected, and in cattle manure, F2, F3, and F4 fractions were not detected [23]. Similar to Ni, F3 and F4 fractions of Pb were almost absent in all trials till at the end of the composting process. The speciation of Pb in trials 1, 2, 3, 4, and 5 is given in Figs. 3?7, respectively, during the composting
process. In trials 1 and 5, F1, F2, and F5 fractions of Pb were increased (percentage of total fraction) during the composting process. This might be due to the mass loss and poor formation of humic substances during the process. In trials 2, 3, and 4, F1 and F2 fractions of Pb were decreased, but the F5 fraction was increased from the total fraction during the composting process. During the course of composting, the thermophilic phase showed intense microbial decomposition of organic matter with the release of Pb from the organic site. A similar observation was also reported during the composting of sewage sludge by Amir et al. [15]. Both F1 and F2 fractions contributed <5% of the total fraction of Pb in all trials. After composting, the F5 fraction of Pb was still the dominating fraction in all trials. In this study, the mobility of Pb was decreased during the composting process; a similar result was also observed by Wong and Selvam [8]. Lead occurs mainly in carbonate form which is moderately mobile form; however organically bound form is potentially available for plants even it is less mobile form [34]. BF of Pb in trials 1 and 5 was increased from 0.037 and 0.034 (initial) to 0.040 and 0.041 (final), respectively; on the other hand, in trials 2, 3, and 4, the BF was decreased from 0.045, 0.045, and 0.043 (initial) to 0.035, 0.017, and 0.013 (final), respectively, during the composting process (Fig. 8). The higher reduction in BF of Pb was observed in trial 4 (70.2%), followed by trial 3 (61.6%) and trial 2 (22.0%) during the process. Reduction in BF of Pb could be explained as when a slightly alkaline medium decreases the F1, F2, F3, and F4 fractions of Pb by forming a Pb-organic matter complex, but the higher alkaline conditions can have a reverse effect on Pb stability due to the amphoteric nature of Pb. However, at pH >11?12, it might form soluble hydroxide complexes that can increase Pb mobility [26]. Significant differences in F1, F2, and F5 fractions of Pb were observed in all trials (
The order of different fractions of Cd in the water hyacinth, sawdust and cattle manure is as follows: F5 (96.9%) > F1 (3.0%) > F2 (0.2%); F5 (98.7%) > F1 (1.3%); and F5 (92.1%) > F1 (7.9%), respectively. The F5 fraction of Cd was predominant in all raw materials. The F3 and F4 fractions were absent in raw materials from the initial to the final compost. The concentration of Cd was very less in cattle manure, but the percentage of F1 fraction was higher than the other two materials [23]. The speciation of Cd in trials 1, 2, 3, 4, and 5 is given in Figs. 3?7, respectively, during the composting process. The F1, F2, and F5 fractions of Cd in trials
1, 2, 3, and 5 were increased from the total fraction of Cd during the composting process. In trial 4, the F5 fraction of Cd was increased, but F1 and F2 fractions were declined with the composting age. One possible explanation for the reduction of F1 and F2 fractions and an increase in F5 fraction in trial 4 may be due to the ability of Cd to chemically bond strongly with organic materials [35]. The decrease in F1 and F2 fractions of Cd could be due to the increase of pH during the composting process (from 5.3 to 7.4). A similar observation was also reported during the composting of sewage sludge by Hanc et al. [36]. Additionally, Cd was directly bound to two or more organic functional groups, mainly carboxylic, carbonyl and phenolic, so that the ion was immobilized in a rigid inner-sphere complex. The F1 and F2 fraction indicates that Cd can be available for plant uptake. The BF of Cd in trials 1, 3, and 5 was increased from 0.032, 0.005, and 0.033 (initial) to 0.038, 0.007, and 0.034 (final), respectively; however, in trials 2 and 4, the BF was declined from 0.012 and 0.045 (initial) to 0.010 and 0.019 (final) during the composting process (Fig. 8). The higher reduction in BF of Cd was observed in trial 4 (56.4%), followed by trial 2 (8.62%) during the process. The reduction in BF could be attributed to the following: most bioavailable fractions (F1 and F2) were bound to two or more organic functional groups, mainly carboxylic, carbonyl, and phenolic, so that the ion was immobilized in a rigid inner-sphere complex [29]. The maximum reduction of BF was observed in trial 4. Significant differences in F1, F2, and F5 fractions of Cd were observed in all trials (
The order of different fractions of Cr in the water hyacinth, sawdust and cattle manure is as follows: F5 (82.7%) > F1 (7.9%) > F4 (4.8%) > F2 (3.8%) > F3 (0.9%); F5 (59.2%) > F1 (31.6%) > F4 (4.5%) > F2 (3.4%) > F3 (1.3%); and F1 (34%) > F5 (31.4%) > F4 (28.3%) > F2 (4.2%) > F3 (2.1%), respectively. The F5 fraction of Cr was predominant in water hyacinth and sawdust; however, in cattle manure, the F1 fraction was predominant. The concentration of Cr was very low in cattle manure, but the percentage of the F1 fraction was higher than that in the other two materials [23]. The speciation of Cr in trials 1, 2, 3, 4, and 5 is given in Figs. 3?7, respectively, during the composting process. In trial 1, F1 and F2 fractions of Cr were decreased, but F3, F4, and F5 fractions were increased from the total fraction during the composting process. In trials 2, 4, and 5, all movable fractions (F1?F4) of Cr were decreased, but the F5 fraction was enhanced with regards
to the total fraction at the end of composting. The significant reduction in fractions F1, F2, F3, and F4 from 10.6%, 3.2%, 6.2%, and 28.1% to 5.3%, 1.9%, 1.1%, and 5.9% of total fraction was observed in trial 4 compared to the other trials. It could be explained that, the F1 and F2 forms might bind with various organic functional groups present in humic substances, while the F3 and F4 fractions might be converted into the F5 fraction during the composting process. The BF of Cr in trials 2, 3, 4, and 5 was reduced from 0.63, 0.53, 0.48, and 0.36 (initial) to 0.36, 0.43, 0.14, and 0.24 (final), respectively; however, in trial 1, the BF was increased slightly from 0.24 (initial) to 0.25 (final) during the composting process (Fig. 8). The higher reduction in BF of Cr was observed in trial 4 (70.5%), followed by trial 2 (42.6%), trial 5 (34.4%) and trial 3 (18.5%) during the process. The reduction in BF of Cr could be explained as follows: F1 and F2 forms may bind with various organic functional groups present in the humic substances, whereas F3 and F4 fractions might be converted into the F5 fraction during the process. Furthermore, the maximum reduction of BF was observed in trial 4, which could be explained by how the addition of appropriate proportion of cattle manure can reduce the Cr availability by increasing humic substances. Significant differences in F1, F2, F3, F4, and F5 fractions of Cr were observed in all trials (
3.2.3.Comparative study of heavy metal speciation in different materials and methods
A similar study was carried out by Singh and Kalamdhad [23] in agitated pile composting of water hyacinth, and reported that the BF of Zn, Cu, Mn, Fe, Ni, Pb, Cd, and Cr were reduced to about 36.4%, 55.5%, 27.2%, 18.8%, 46.9%, 55.6%, 10.5%, and 36.4%, respectively, from initial to final compost in trial 4. However, in the present study, the BF was reduced by approximately 37.5%, 58.7%, 29.6%, 32.2%, 65.0%, 70.2%, 56.7%, and 70.5% for Zn, Cu, Mn, Fe, Ni, Pb, Cd, and Cr, respectively, during the composting process (Fig. 5). The higher reduction in BF of all metals was examined in rotary drum composting compared to agitated pile composting of water hyacinth. The higher reduction in BF justifies the reduction of bioavailable fractions (F1, F2, F3, and F4) and enhanced metals into the inert fraction (F5 fraction) in rotary drum composting. The present study reveals that the rotary drum was more proficient for BF reduction compared to the agitated pile. The higher reduction of BF in the drum might be due to a higher reduction of organic matter and conversion into humic substances compared to the agitated pile. Walter et al. [10] concluded that the availability of Cu and Cd was increased during the composting of sewage sludge in the pile; however, in the present study, the availability of these metals was reduced significantly. Nomeda et al. [37] concluded that the bioavailability of Mn, Pb, and Zn increased after sewage sludge composting in the pile, but in the present study, the bioavailability of these metals was reduced.
The speciation of heavy metals in the final compost affected the properties of the initial compost materials, composting process and physicochemical properties of the final compost, similar to TOC and pH. The mobility and bioavailability of heavy metals were reduced significantly during rotary drum composting process compared to the traditional methods. The total concentration of Cu, Cr, and Cd was very low compared to the other metals (Zn, Mn, Fe, Ni, and Pb); however, the percentage of exchangeable and carbonate fractions of these metals were similar to other metals. The total concentration of Cu, Cr, and Cd was approximately similar, but the exchangeable and carbonate fractions of Cr was twofold than the Cu and fifty fold than the Cd, which indicates that Cr is more available for plants and is toxic to the environment. The order of BF of different metals in the compost was Mn (0.93) > Fe (0.66) > Zn (0.47) > Cr (0.43) > Cu (0.35) > Ni (0.042) > Pb (0.041) > Cd (0.02). The order of BF indicates that the toxicity of metals does not depend on its total metal concentration. The total concentration of Pb was higher than Zn, Cu, Mn, Ni, Cd, and Cr, but its BF was lowest among all eight metals except Cd. The reducible and oxidizable fractions of Ni, Pb, and Cd were not found during water hyacinth composting, but on the other hand, exchangeable and carbonate fractions were found in all trials, such as more toxic and easily bioavailable fractions.