The molecular existence of drug particles has been presumed to have a role in the formation of ligand moiety to accelerate or decelerate cellular/biochemical processes. However, certain remedies used in homeopathy are ultra-highly diluted (above 12C centesimal potency) by using the homeopathic procedure of dilutions and agitations and are not theoretically expected to contain even a single molecule of the original drug bulk substance, thus making the reported abilities of these highly-diluted remedies to cure or ameliorate disease symptoms suspect [1, 2]. Even though most clinical research conducted on homeopathic medicines has shown positive results [3-5], rationalists often refuse to accept even scientifically observed facts, apparently being bound by the limits of existing knowledge.
Earlier, many hypotheses were put forward relating to the efficacy of homeopathic remedies, but those soon became obsolete due to insufficient validation. Later thought was succussion-mediated release of silicon and silica precursors from the interior of the glassware into solution. Later the nano-silica theory was evolved [6, 7], but even though nano-silica has a fair ability to stimulate immune responses, the workings of nano-silica are not specific to a given homeopathic remedy; it can serve as a non-specific carrier and biological amplifier of effects.
Recently, many researchers with an open mind have been quite prone to believing in the possible efficacy of ultra- high dilutions ever since the presence of nano-sized solvent super-structures in ultra-molecular dilutions [8- 10] was discovered. The concentrations of the starting materials, even though at extremely low (pictogram/milliliter) levels, were shown not to decrease as expected with serial dilutions, but instead to form an asymptote beyond 6C potency (dilution factor of 1012) [11]. A recent finding pointed out that homeopathic succussion procedures could break down larger plant particles into small nanoparticles, just as sonication can break down plant starch into nanoparticles [12]. Thus, ultra-high dilutions, demonstrations of their biological actions and understandings of their mechanisms of action have again become subjects of serious scientific exploration.
Much research has shown the biological activities (http:// www.carstens-stiftung.de/hombrex/index.php) of ultra- highly-diluted drug materials; further, their capability to modulate different signal genes and proteins was also identified [13]. Therefore, Khuda-Bukhsh [14] and Khuda- Bukhsh and Pathak [17] advocated a working hypothesis claiming that the working principle of the homeopathy remedy should be based upon a switch on/off mechanism of gene expression, particularly by causing epigenetic modifications.
The equilibrium of histone acetylation can be controlled by using histone acetyltransferase and histone deacetylase (HDAC) enzymatic activities. Their balance seems to be essential for normal cell growth while imbalance is often associated with carcinogenesis and cancer progression [18]. The present study was, therefore, designed to investigate if Condurango 30C, an ultra-highly-diluted homeopathic remedy, could demonstrate its anti-cancer potential in terms of its ability to modulate histone deacetylase activity (HDAC) in the cervix cancer cell line HeLa, an accepted protocol to evaluate gene regulatory activities. The HeLa cell line was particularly chosen for this study as HDAC activity (both HDAC1 and HDAC 2) is reported to be typically high in this cancer cell line, so its modulation, if any, by using a drug can be clearly demonstrated.
HeLa cells were procured from the National Centre for Cell Science, India, were maintained in a CO2 incubator at 5 % CO2 level under ambient O2 and humid conditions, and were cultured at 37℃ in DMEM (Dulbecco’s modified Eagle medium) containing 10% heat-inactivated FBS (fetal bovine serum)and 1% antibiotic mixture. Condurango 30C was procured from HAPCO, India. Preparation of Condurango 30C was made according to homeopathic principles of dilution and succussion. One ml of ethanolic mother tincture of Condurango was added to 99 ml of 65% ethanol and given 10 mechanical jerks to bring it to potency 1. One volume of this solution of potency 1 was again added to 99 volume of 65% ethanol and given 10 jerks to bring it to potency 2 and so on [15]. The potency 30C had, thus, been diluted 10-60 times before use.
HeLa was dispensed in 96-well flat-bottom microtiter plates (Tarsons, India) at a density of 1×103 cells per well, treated with various concentrations of Condurango 30C (0%-6%) and incubated for 48 h. Thiazolyl blue tetrazolium bromide (MTT) was then added to the well at a concentration of 10 μM, and the plates were incubated for 2-3 h at 37℃in the dark. To stop the reaction and develop a blue color, we used acidic isopropanol, and the OD (optical density) was taken at 595 nm by using a microtiter plate reader [19].
Condurango 30C doses were selected for the MTT assay experiment. In all the experiments, other than immuno- blotting, HeLa cells were treated with 2% Condurango 30C for different time periods (0, 3, 6, 9, 12, 15, 18, 21, and 24 h). For the control sets, a 2% vehicle control (placebo) from the same stock of alcohol that had been used by HAPCO, Kolkata, to prepare the drug was maintained while for the immuno-blotting study, three doses were considered, 2%, 3% and 4% drug, and the highest alcohol dose (4%) was taken for the purpose of serving as the placebo control.
Cells were incubated in serum-free media in the presence or absence of SB (sodium butyrate; a histone deactylase inhibitor) and Condurango 30C (2%) for different intervals (0, 3, 6, 9, 12, 15, 18, 21, and 24 h). Nuclear lysate was prepared, and total HDAC activity was measured according to the manufacturer's instructions (Upstate Millipore, USA). Nuclear extract was prepared from placebo (2%)- and Condurango 30C (2%)-treated cells and then used for the quantification of HDAC1, HDAC2 and STAT3 by using the indirect ELISA method according to the method described by Cell Signalling Technology, USA. p-nitro phenyl phosphate (p-NPP) was used as a color developer, and color intensity was measured at 405 nm with respect to a blank [20].
Fifty μg of nuclear proteins and cellular proteins were isolated from the placebo-control (4%)- and the drug-treated cells to determine the activities of HDAC1, HDAC2 (from nuclear extract) and p53, p21, Akt, GAPDH (from total cellular extract). Bands were developed by using the conventional western blotting technique, and densitometry was quantified by using ‘image J’ software (National Institute of Health, USA) [21].
To analyse the cell cycle, HeLa cells were treated with Condurango 30C (2%) for different time-periods (0, 12, 24, 36 and 48 h); then, treated and control (2% placebo) cells were fixed in 70% chilled ethanol. Fixed cells were then made RNA free by incubating in 10-mM RNase for 10 min in the dark at 37℃ and stained by using propidium iodite (10 μM) for 20 min. Fluorescence intensities were determined by using a flow cytometer with a FL-2A filter. Data were analysed by using Cyflogic software [22].
Observers were “blinded” during observational part of the experiments. The drug and placebo samples were coded and the codes were later deciphered for data analysis. Statistical analysis was performed by using one-way ANOVA with LSD post-hoc tests and SPSS.20 software to identify whether differences among the mean values of the different groups were significant. Results were expressed as means ± SEs (standard errors). A value of
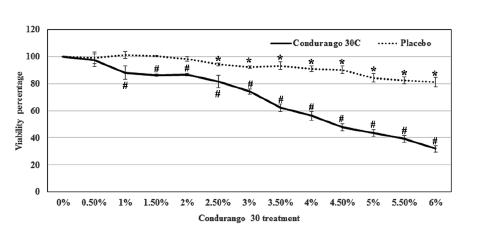
Condurango 30C treatment reduced the viability of HeLa cells in a dose-dependent manner. At 0.5% dose of Condurango 30C, the cells’ viability was 97.4 ± 4.8%, and it was reduced to 31.9 ± 2.4% by the application of 6% Condurango 30C. The 50% cell death was achieved at a 4.6 ± 0.1% of Condurango 30C treatment (Fig 1) To check if the cytotoxicity generated by Condurango 30C was due to ethanol, we checked the viability of HeLa with it. The viability of cells after 48 h treatment, even after 6% alcohol treatment, was 81.2 ± 3.4 (Fig 1) This finding demonstrates that Condurango 30C is capable of reducing the viability of HeLa and that the cell death was not due to only an alcohol effect.
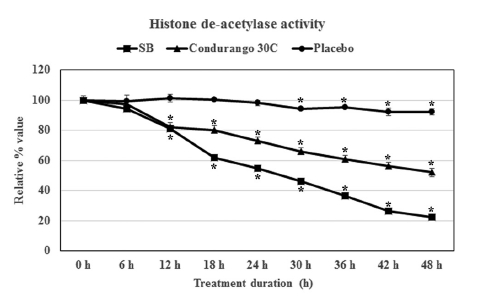
We compared the inhibitory effect of Condurango 30C on histone deacetylase enzyme with that of 5-mM SB treated cells (SB being a known inhibitor of HDAC activity) and with that of ethanolic-placebo- treated cells. A time-dependent study revealed that treatment with SB drastically reduced the HDAC activity after 18 h of treatment, and it was reduced to 22.3 ± 1.8% at 48 h (Fig 2) Condurango 30C was also able to reduce HDAC activity, and a linear reduction pattern was observed. The activity was reduced to 52.0 ± 2.6% after 48 h of treatment, but in case of the placebo, the HDAC activity was 92.1 ± 2.0% (Fig 2) These findings clearly demonstrated that ultra-highly-diluted Condurango 30C had the modulatory effect on histone deacetylase enzyme activity, thereby showing its potential to control gene expression, while the placebo lacked this ability.
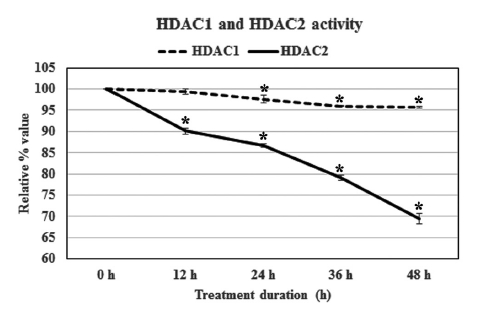
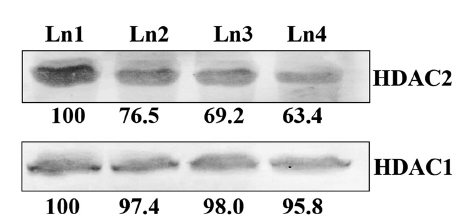
The result revealed that Condurango 30C could only modulate HDAC2 activity, but not HDAC1 activity. The relative percentage values of HDAC2 activity sharply declined after 24 h treatment, and at 48 h, the activity of HDAC2 was 69.4 ± 1.2%. Thus, the modulatory effect of Condurango 30C on HDAC1 was relatively low; the treatment could reduce the activity of HDAC1 only to 95.7 ± 0.1% after 48 h of incubation (Fig 3)
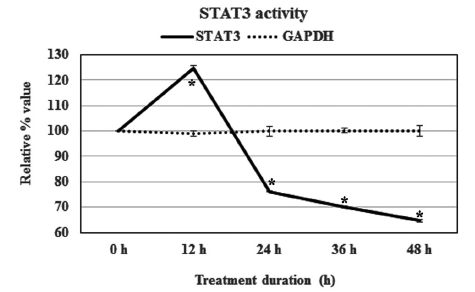
Condurango 30C could modulate STAT3’s expression after treatment. At the 12-h point, the STAT3 activity was up-regulated by 24%, but the relative expression of this transcription factor was reduced for longer times. The activity values were determined to be 76.0 ± 0.1, 70.1 ± 0.3 and 64.7 ± 0.5% at 24, 36 and 48 h of treatment, respectively (Fig 4)
Immuno-blotting assay revealed that no palpable change in the expression of HDAC1 protein because of drug treatment, but compared to the placebo control, the expression of the other histone deacetylase modulatory protein, HDAC2, was down-regulated by using the Condurango 30C 30C treatment (Fig 5)
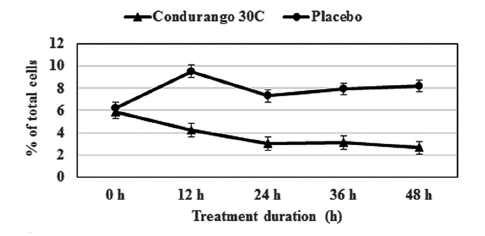
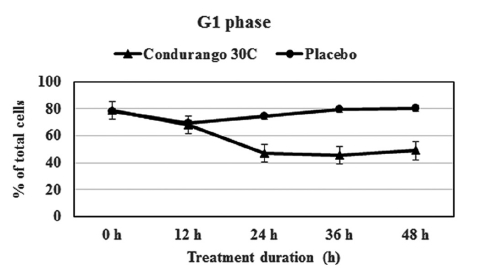
The flow-cytometry data clearly indicated a gradual decrease in cell population at the S-phase in a time-dependent manner with respect to the control, suggesting a reduction in cellular DNA synthesis (Fig 6) This provided evidence for a re-coiling of DNA in treated cancer cells. Here, histone the deacetylase enzyme might be playing a role in reducing DNA synthesis. On the other hand, a time-dependent increase in cell population at the G1- stage by using Condurango 30C treatment (Fig 7) was noted. This might be evidence for an initiation of differentiation/ cell death via apoptosis.
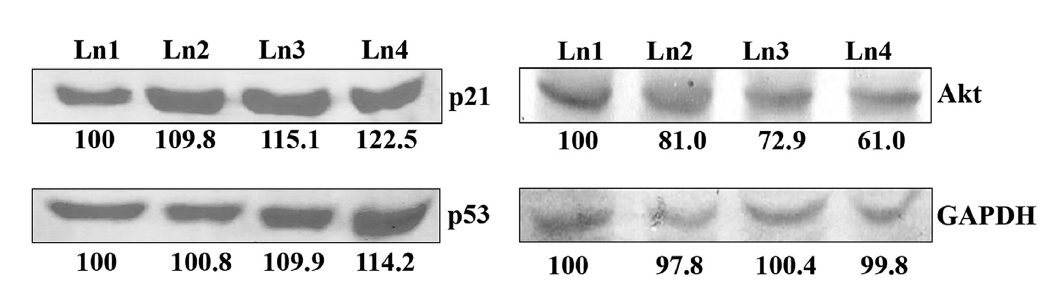
p21 and p53 are two tumour suppressor genes, which are activated when cell cycles are arrested by any external agent. With Condurango 30C-treatment, the expressions of these two proteins were elevated in dose-dependent manner (Fig 8) The expression of another growth-related protein, Akt, was down-regulated by drug treatment (Fig. 8), thereby suggesting an inhibitory effect of Condurango 30C on cell proliferation.
Our earlier works [14-17] revealed quite clearly that certain ultra-highly-diluted homeopathy remedies were capable of altering gene expressions while the succussed alcohol (placebos) invariably failed to do so; however, the precise molecular mechanisms behind successful gene alterations caused by using potentized homeopathic remedies still remain largely unknown [15]. Therefore, the present findings are quite significant because we have demonstrated for the first time that an ultra-highly-diluted remedy can have affect one of the key regulatory machineries of gene expression, namely, the acetylation/deacetylation mechanism of histone core protein. HeLa cells, known for their high level of HDAC activity, can actually serve as a model system for testing the ability of any drug in epigenetic modification because any agent that can alter the process of deacetylation can also be considered as having a significant and direct role in the regulation of the gene expression [23]. In this study, ultra-highly-diluted Condurango 30C showed a potential for modulating the histone deacetylase enzyme. Correspondingly, another known fact is that when an agent is capable of suppressing deacetylation activity, it can also block the synthesis of nuclear DNA [24]. In this study, low-dose Condurango 30C treatment lowered the cell population at the S-phase, thus indicating a reduced synthesis of DNA.
Imbalances in the equilibrium of histone acetylation are also associated with carcinogenesis and cancer progression [25]. Tumor cells generally show a high level of HDAC activity [26], and when this activity can be blocked or suppressed, a corresponding check on cell growth and proliferation follows. In our study, we found that Condurango 30C clearly suppressed HDAC2 activity while HDAC1 activity remained almost unaffected, signifying that although HDAC1 and HDAC2 are closely-related proteins sharing about 85% homology, they may possibly be regulated through different pathways. Some recent studies have shown that certain specific inhibitors of class I HDACs, like valproate [27] and trichostatin [28], also selectively affect HDAC2, showing the sensibility of this enzyme to be more delicately poised than other groups of HDAC enzymes.
HDAC2, a key regulator of genes, cell cycle, apoptosis, cell adhesion and migration, belongs to the class I histone deacetylases [29]. HDAC2 acts as a transcriptional repressor through the deacetylation of lysine residues present at the N-terminal tail of histone proteins (H2A, H2B, H3 and H4) [30]. The deregulation/over expression of HDAC2 activity has been linked to tumor/cancer development, including colon, gastric, cervical and prostate carcinomas [31]. HDAC2 overexpression is implicated in cancer partly through its aberrant recruitment and consequent silencing of the tumor suppressor genes p21 and p53, which are associated with histone acetylation at the promoter region, which processes can be reversed by the treatment with HDAC inhibitors [32]. HDAC2 also regulates gene expression through the deacetylation of specific transcription factors, including STAT3 [33]. Our experiments with the model cell line HeLa showed that Condurango 30C initiated HDAC2 reduction and affected cell differentiation by its ability to arrest cells at G1. This further testifies that Condurango 30C treatment can also induce cellular differentiation in HeLa cells. The reduction in cell viability may, thus, result more from a reduction in cell number than from an inhibition of cell proliferation and induction of cell differentiation caused by Condurango 30C treatment.
The increase in p21WAF1 protein after HDAC2 suppression caused by using Condurango 30C suggests that HDAC2 may be involved in the regulation of p21WAF at the transcriptional level. On the other hand, cellular differentiation and cell-cycle control are associated with an increase in p21WAF expression [27]. Also, being a cyclin-dependent kinase inhibitor, p21WAF can influence G1 progression in both positive and negative manners [34]. Thus, the level of p21WAF in the cell may be an important determining factor as to whether the cell differentiates or undergoes apoptosis. For example, lower levels of p21WAF may cause the cell to differentiate, and higher levels may direct the cell to an apoptotic pathway [35]. However, the reason p21WAF was found to be up-regulated after suppression of HDAC2 activity by Condurango 30C in our study, leading to apoptosis, could not be properly determined.
As p53 is a known regulator of p21WAF expression, we determined the level of p53 protein in the cells after HDAC2 suppression caused by Condurango 30C. On the other hand, cell-cycle arrest and growth inhibition may induce a greater sensitivity to the cell of having more tumor suppressor proteins like p53 [35]. Along with cell-cycle arrest and growth inhibition, p53 expression was up-regulated. With the arrest of growth, the expression of Akt, an important cell differentiation and proliferative molecule, was down-regulated. One hypothesis to explain this could be that a p53-Akt crosstalk took place after the drug’s induction.
The results of the present study testify to the ability of ultra-highly-diluted homeopathic remedies to modulate deacetylase activity, which further vindicates the hypothesis [13-15] that ultra-highly-diluted homeopathic remedies act by triggering a gene regulatory mechanism in a chain (cascade) reaction. Presumably, this is due to the existence of nano-particles of the drug materials still present in the ultra-highly-diluted remedies.