Pharmacopuncture is a new form of acupuncture treatment combining acupuncture and herbal medicine. With acupuncture being based on the meridian theory and herbal medicine being based on Qi and flavor theory, Pharmacopuncture utilizes both meridian and Qi/flavor theories. Pharmacopuncture is a unique treatment of traditional Korean medicine using needling and herbal medicine based on Qi-flavor theories and acupoint selection based on meridian theories [1]. In addition, eight-principle pharmacopuncture (EPP) is a method of treating disorders by using the founding principles in Korean medicine of Yin/Yang, internal/external, cold/heat, and deficiency/excess [1].
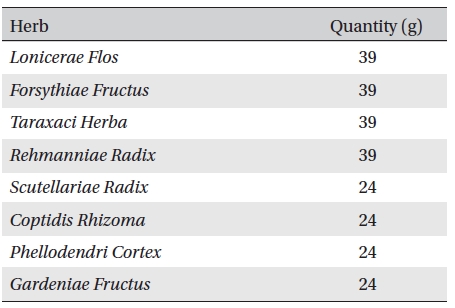
Anti-inflammatory pharmacopuncture (AIP), which is a heat-clearing and detoxifying drug, is composed of Lonicerae Flos, Forsythiae Fructus, Taraxaci Herba, Rehmanniae Radix, Scutellariae Radix, Coptidis Rhizoma, Phellodendri Cortex and Gardeniae Fructus [2]. AIP has been widely applied to musculoskeletal diseases in Korean medical clinics, and excellent effects on pain relief have been announced recently [3-5]. Shin
Anti-inflammatory pharmacopuncture is composed of Lonicerae Flos, Forsythiae Fructus, Taraxaci Herba, Rehmanniae Radix, Scutellariae Radix, Coptidis Rhizoma, Phellodendri Cortex and Gardeniae Fructus (Table 1) [2]. A distillation method was used for extracting EPP. Herbs were washed and sliced for easier extraction. The herbs were then placed in the bottom of a flask that was then filled with tertiary distilled water, soaked and boiled. The liquid was collected from the upper part of the distillation apparatus. The pH and the concentration of the extract were measured, followed by filtration, subdivision and sterilization [1]. The pharmacopuncture was made in a sterile laboratory by using steam distillation in a laboratory at the Korean Pharmacopuncture Institute (KPI) according to the following procedure: Cleansing → Leaching → Extracting → Distilling → Cooling → pH, NaCl control → Filtering → Sterilizing → Packing [9]. The pharmacopuncture was stored in a refrigerator (2-8℃).
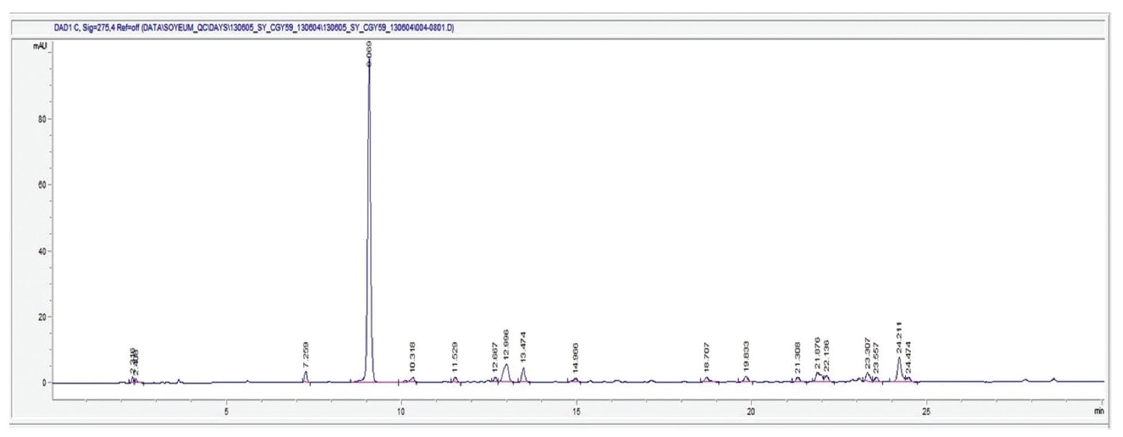
The HPLC (High performance liquid chromatography) Agilent Technologies 1260 infinity equipment was used for the analysis, and the analysis conditions were a column of Capcell Pak C18 (type: UG80 5 ㎛, size: 4.6 ㎜ I.D. x 250 ㎜, Shiseido), a 1-㎖/min flow, a column temperature of 35℃, a sample dose of 50 ㎕, and UV 275 ㎚. For the mobile phase condition, solvent A, H2O (5-mM NH4OAc), and solvent B, CAN (5-mM NH4OAc), were used to perform the analysis under gradient conditions in which mobile phase A was changed from 97% to 10% in 40 min. The result of HPLC analysis on AIP under these conditions is shown as a chromatogram in (Fig 1) In that chromatogram, the peak of the 9.0-min section was highest compared to the other peaks, and the ratio of the peak area of the 9.0-min section to the total area was found to be 60%. AIP is expected to contain an aromatic substance, and a qualitative analysis study is deemed necessary to determine the unknown components associated with the peaks detected.
Twenty-four (24) male and 24 female rats were purchased from ORIENTBIO INC. The 24 male and the 24 female rats were 5 weeks old with body weights of 121.8~143.4 kg, and 103.8~120.9 kg, respectively. The laboratory conditions were as follows: temperature: 21.0-23.1℃, relative humidity: 45.7%-61.5%, 10-15 ventilations/hr, lighting cycle of 12 hr/day and illuminance of 150-300 Lux. The rats were supplied with food (Teklad Certified Irradiated Global 18% Protein Rodent Diet 2918C) and clean water (tap water of Cheongju filtered with a water sterilizer and irradiated with ultraviolet rays). After 7 days of acclimatization, the weights of the male rats were 183.0-205.6 g, and those of the female rats were 141.9-160.0 g. The 6-week-old Sprague-Dawley rats were grouped by using the criteria of their weights being close to the mean weight. A total of 20 male rats and 20 female rats were distributed into 4 groups with 5 mice of each sex per group. The remaining animals were excluded from the test.
[Table. 1] Contents of anti-inflammatory pharmacopuncture
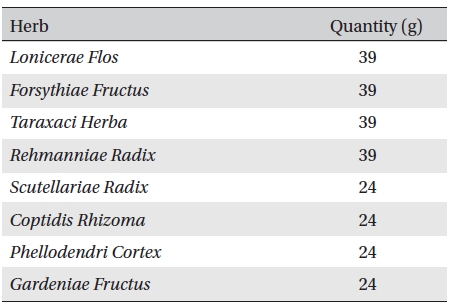
Contents of anti-inflammatory pharmacopuncture
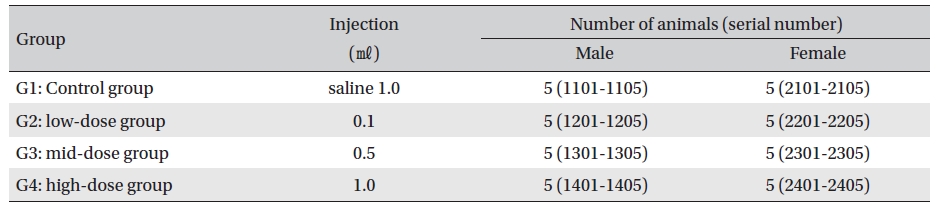
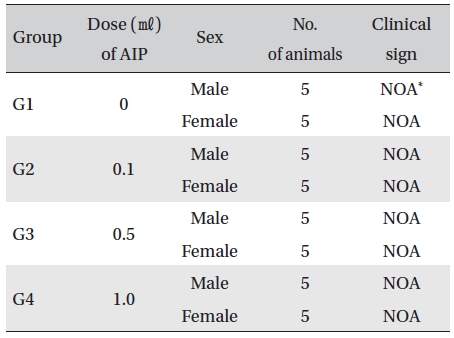
No deaths were observed as a result of single intramuscular- dose in the male or the female rats during preliminary test (Biotoxtech Study No.: B12868P). Based on those results, 1.0 ㎖/animal was selected as the high-dose, and 0.5 and 0.1 ㎖/animal as the mid-dose and the low-dose, respectively. Normal saline solution, 1.0 ml/animal, was used for the control group (Table 2)
Injection was performed using a disposable syringe (1 ㎖, 26G). In G2, 0.1 ㎖ of AIP was injected through IM at one point on the left thigh muscle. In G3, 0.5 ㎖ of AIP was injected through IM as in G2. In G4 and G1, injection was done in the left and the right thigh muscles at 0.5 ㎖/site. An intramuscular dose was selected because AIP should be clinically applied to muscles. This study was conducted under the approval of the Institutional Animal Ethics Committee.
Body weights, hematology, and clinical chemistry test results were tested using SAS (version 9.2, SAS Institute Inc., U.S.A). The Bartlett test was performed for homoscedasticity (significance level: 0.05). In the case of homoscedasticity, a one-way analysis of variance (ANOVA) was performed to yield significance (significance level: 0.05), and multiple testing of Dunnett’s
[Table. 2] Grouping of the rats
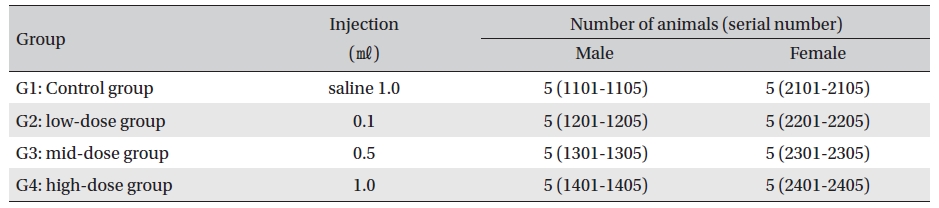
Grouping of the rats
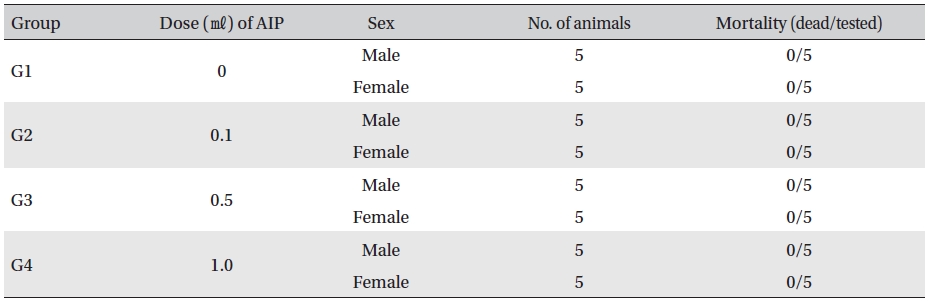
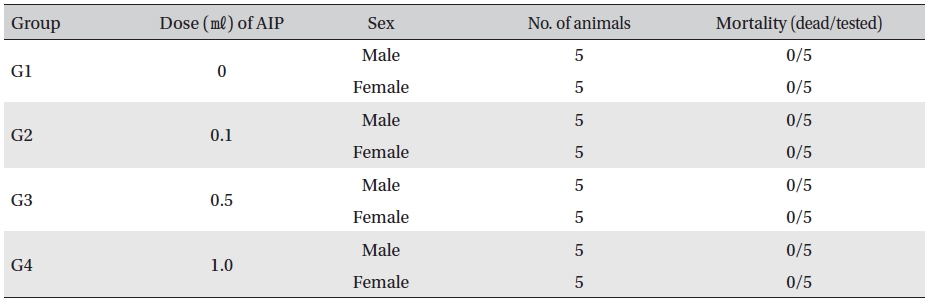
Mortality
No mortalities occurred in any group (Table 3) In addition, on the day of injection (Day 0), clinical symptoms (type of toxicity symptom, time of expression, time of recovery, etc.) and fatality were observed at 30 min, 1, 2, 4 and 6 hr after injection. From Day 1 to Day 14, general symptoms were observed once a day. As a result of observations until Day 14 after injection of AIP, no abnormalities were found in any of the experimental rats (Table 4)
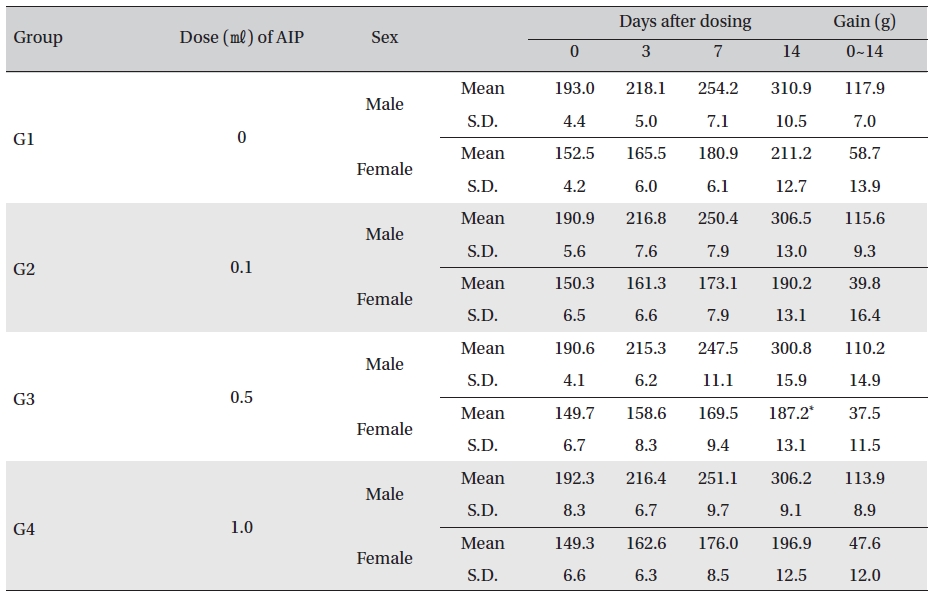
Body weight was measured on the day of injection (before injection) and on Days 3, 7 and 14. All groups showed continued increase in body weight after selection of the experimental groups. No significant differences existed among the experimental groups with the exception of a significant difference between female rats in G1 and G3 14 days after dosing. However, it was an adventitious change with no dose dependance. No hanges caused by the test substance were recognized (Table 5)
[Table. 4] Clinical signs from Day 0 to Day 14
Clinical signs from Day 0 to Day 14
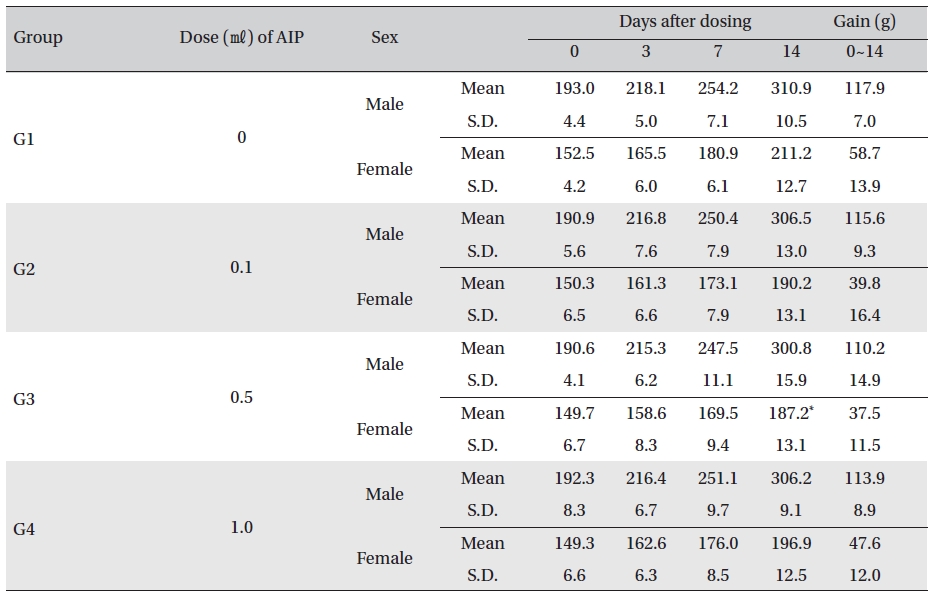
Mean body weights
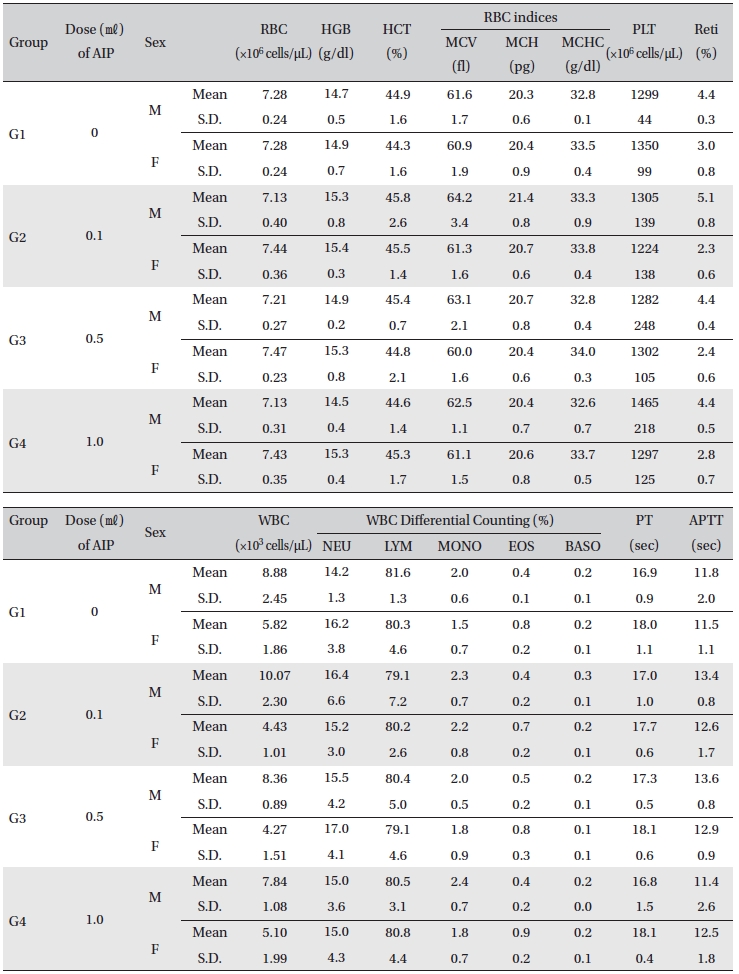
[Table. 6] Mean hematology parameters
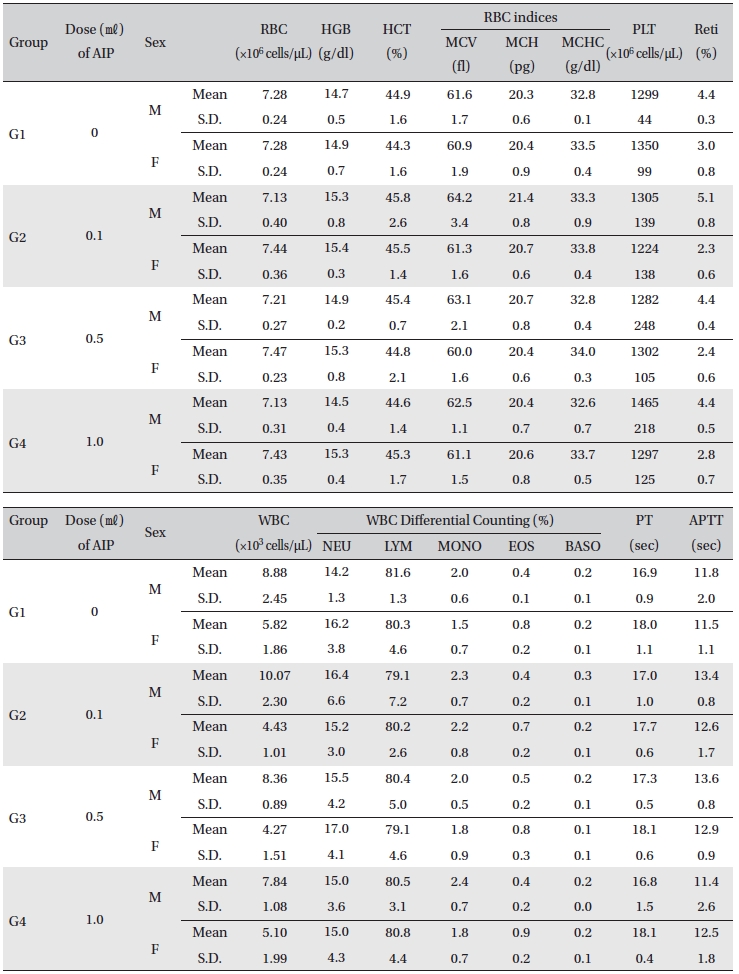
Mean hematology parameters
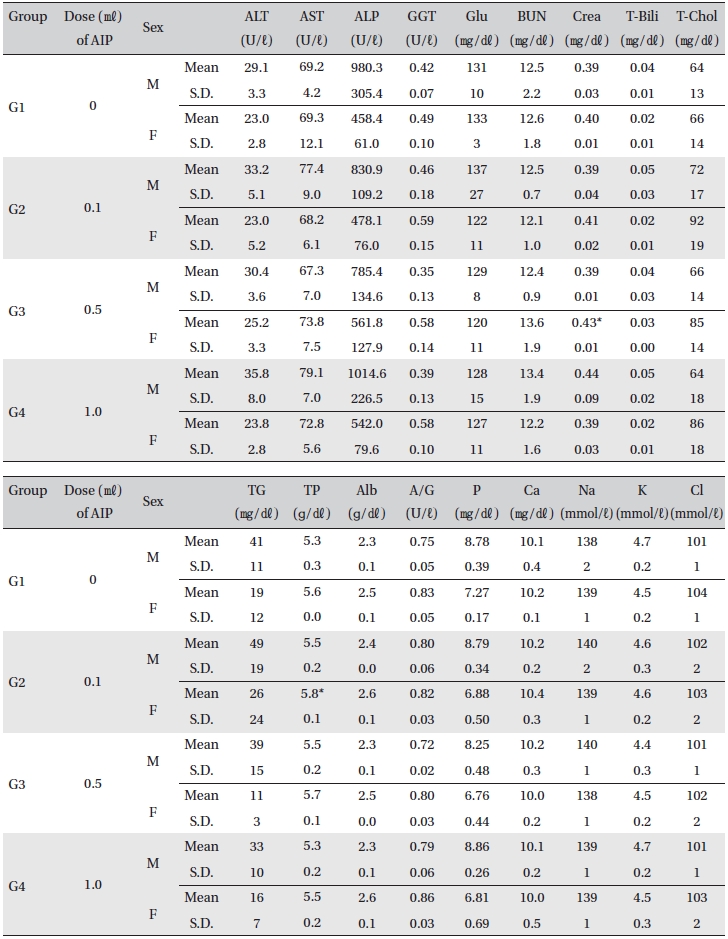
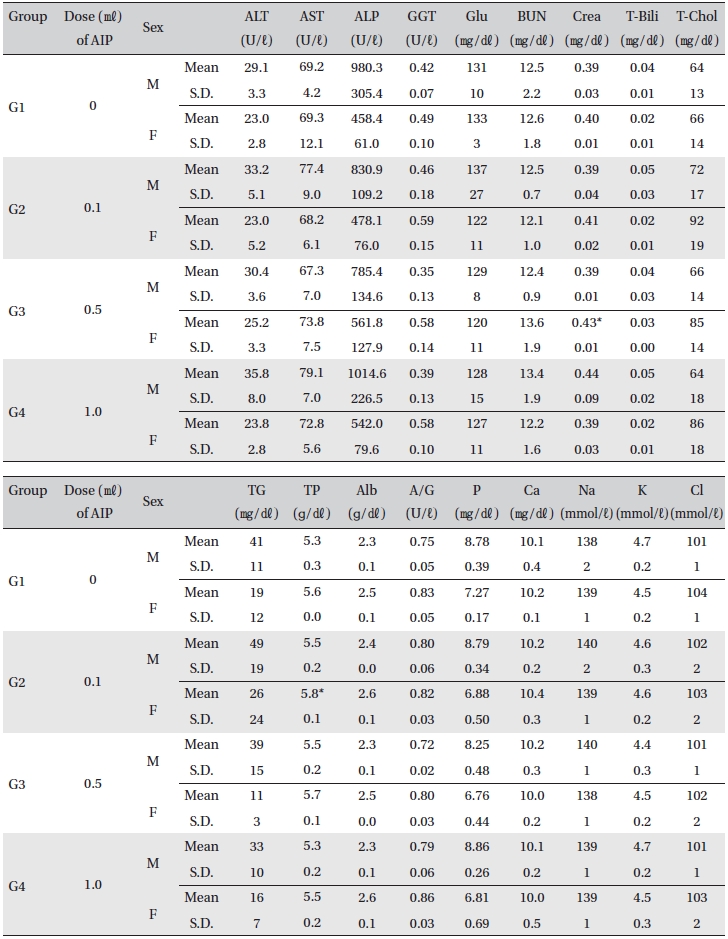
[Table. 7] Mean clinical chemistry
Mean clinical chemistry
Necropsy findings
[Table. 9] Histopathological findings
Histopathological findings
All rats had been fasting more than 18 hr before necropsy. Blood was collected from the abdominal aorta on Day 15 after applying anesthesia with isoflurane. The hematology test was done by using a blood corpuscle analyzer (ADVIA 120, SIEMENS, Germany) after placing about 1㎖ of the collected blood in a tube containing EDTA(Ethylenediaminetetraacetic acid). Erythrocyte count (RBC), hemoglobin (HBG), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet (PLT), leucocyte count (WBC), WBC differential count (neutrophils, lymphocytes, monocytes, eosinophils, basophils) and Reti (reticulocytes) were measured. For the coagulation test, about 2 ㎖ of the collected blood was placed in a tube containing 3.2% sodium citrate and was centrifuged for 10 min at 3,000 rpm before collecting the blood plasma. Measurement was done using a coagulation time analyzer (Coapresta 2000, SEKISUI, Japan). Prothrombin time (PT) and activated partial thromboplastin time (APTT) were measured. The hematology test showed no significant differences between the normal saline group and any of the AIP groups (Table 6)
For the clinical chemistry test, the unused blood remaining after the hematology test was centrifuged for 10 min at 3,000 rpm before blood serum was collected. A clinical chemistry analyzer (7180, HITACHI, Japan) and electrolyte analyzer (AVL9181, Roche, Germany) were used to measure alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT), blood urea nitrogen (BUN), creatinine (Crea), total bilirubin (T-Bili), total protein (TP), albumin (Alb), A/G ratio, total cholesterol (T-chol), triglycerides (TG), phosphorus (P), glucose (Glu), calcium (Ca), chloride (Cl), sodium (Na) and potassium (K). The clinical chemistry test showed no changes caused by the test substance in animals of either sex in any group, except that the total mean protein of G2 females and mean creatinine of G3 females showed statistically significant changes with
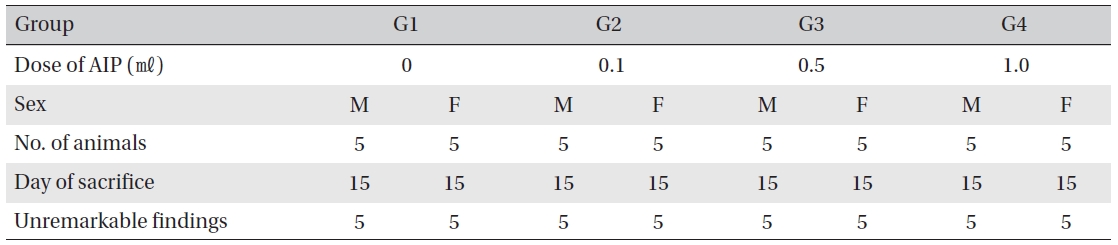
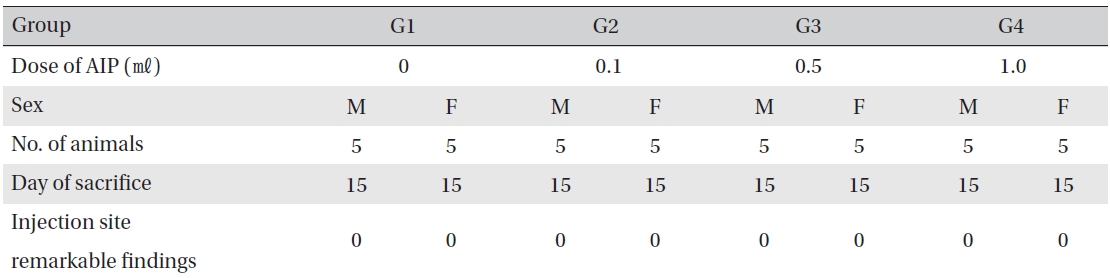
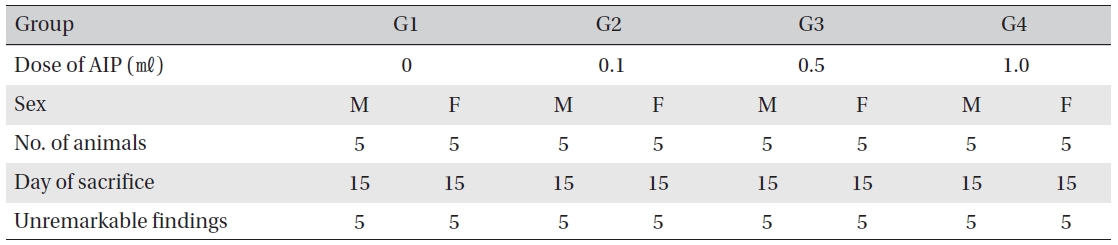
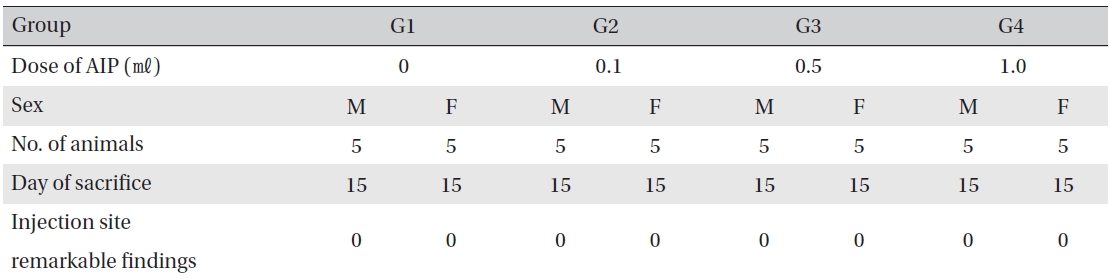
Visual inspection after necropsy showed no visual abnormalities in the male and the female animals in any experimental group (Table 8) In addition, histopathological tests on the injected parts showed no effect due to the test substance (Table 9)
Eight-principle pharmacopuncture is effective when organ symptoms are accurately diagnosed and treated with proper herbal medicine. This pharmacopuncture system does not create tolerance, toxicity, or addiction [1]. The amount of pharmacopuncture injection is divided into the amount for each acupoint and the total amount for the entire treatment. The standard amount is 0.1 ㎖ for each acupoint. The amount that is injected is increased gradually: 0.1 ㎖ on each acupoint on the first day, 0.2 ㎖ on the second day, and 0.3 ㎖ to 0.5 ㎖ on the third day. When 0.5 ㎖ has been injected without any side effects and the symptoms had improved, the dosage is no longer increased. However, if the symptoms persist, pharmacopuncture may be injected up to 1.0 ㎖ under careful supervision for possible side effects. If the improvement is weak, then the dosage can be increased to 1.5 ㎖. After that, increases can be made in increments of 0.5 ㎖, depending on the response. The total amount of injection should be between 3.0 ㎖ and 5.0 ㎖. Even if the patient does not show significant improvement, increasing the amount beyond 5.0 ㎖ must be carefully considered [1].
Side effects are possible when more than an adequate amount is injected, when the medicine and the nature of the disorder are not in harmony and when contaminated/ denatured pharmacopuncture extract is injected. If side effects occur, body ache and pain around the acupoint maybe observed [1].
In this study, an intramuscular-dose toxicity test of AIP was performed by Biotoxtech to evaluate the stability of AIP, which had been manufactured by the KPI according to GLP. The result of single intramuscular-doses of AIP in the 4 groups of rats showed that the approximate lethal dose for both male and female rats exceeded 1.0 ㎖ Therefore, AIP is a relatively safe pharmacopuncture that can be used for treatment, but further studies should be performed in the future.
Conventionally EPP has been confined to using pharmacopuncture doses of less than 1.0 ml, which is the reason in this study for choosing 1.0-1.5 ml as the level of AIP dosing corresponding to the lethal dose. As none of the test subjects were affected by injections of up to 1.0 ml of AIP, it can be assumed that levels over 1.0 ml may have a determinant effect. However, if the exact lethal dose is to be established, further studies are needed. Finally, the results clearly show that AIP dosing up to 1.0 ml is safe.