Diabetes mellitus (DM) is a syndrome of chronic hyperglycemia due to a relative insulin deficiency, insulin resistance, or both. Diabetes is rampant in many parts of the world, with incidence rates increasing yearly. Many chemical agents are available to control and treat diabetes but overall protecting on against long-term complications requires much efforts and money. In addition, the side effects from using chemical hypoglycemic agents, to maintain euglycemia and avoid late-stage diabetic complications disgust most patients. Alternatives to these synthetic agents, plants, provide a potential source of hypoglycemic drugs and are widely used in several traditional systems of medicine to prevent diabetes. The actions of herbal medicines and nutraceuticals are commonly multi-target, multi-channel and synergistic, due to the variety of constituents within a single natural product. Owing to these properties, herbal medicines and nutraceuticals may be beneficial in dealing with diabetes itself, as well as with its complications, because various mechanisms are involved in diabetic vascular complications. [1]. However, because diabetes is a dangerous disease with many potential complications, alternative treatments for diabetes should not be attempted as a substitute for conventional medical care.
The objectives of this study were to examine how an integrated approach to the treatment of type 2 diabetes mellitus could improve glycemic control, how immune-potentiating activities respond to oral hypoglycemic agents, and whether or not botanical compounds have an adjuvant effect among primary care patients.
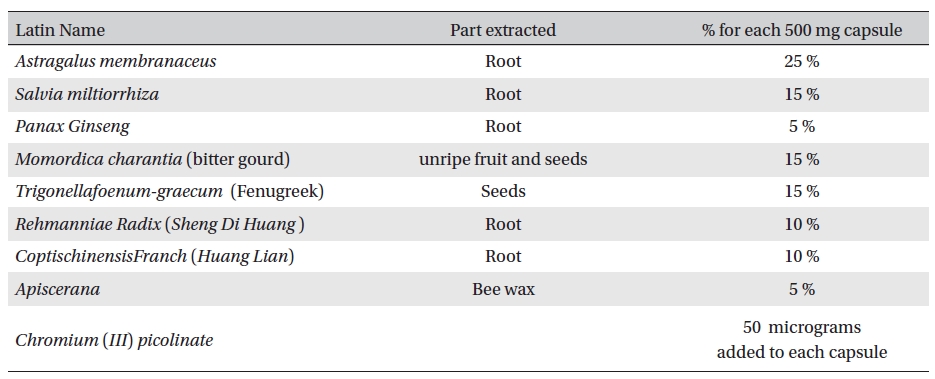
Aqueous extracts of the experimental materials were obtained and mixed in a proper formula. (Table 1) Aqueous extract of all ingredients were purchased from Shanghai Wellong Medical Material CO., LTD. (Shanghai, China), and were identified by the Department of Authentication of Dubai Pharmacy College, Alternative Department.
The vegetable Momordica charantia L. is also known as bitter gourd, balsam pear, bitter melon. Bitter gourd extracts possesses antioxidant, antibacterial, antiviral, anti- hepatotoxic and anti-ulcerogenic properties as well as the ability to lower blood sugar. Animal and in vitro data support both insulin secretagogue and insulin-mimetic activity of the fruit. A polypeptide (p-insulin) produces hypoglycemic effects in humans and animals by subcutaneous injection, but oral activity are questionable. [7-10] Preliminary animal and human trials suggest possible hypoglycemic and anti-hyperlipidemic properties of oral fenugreek seed powder. In animal and several human trials, fenugreek seeds have been found to lower fasting serum glucose levels, both acutely and chronically. The hypoglycemic effects of fenugreek have been attributed to several mechanisms. [11-15]
[Table. 1] Ingredients of the botanical compound used in this study
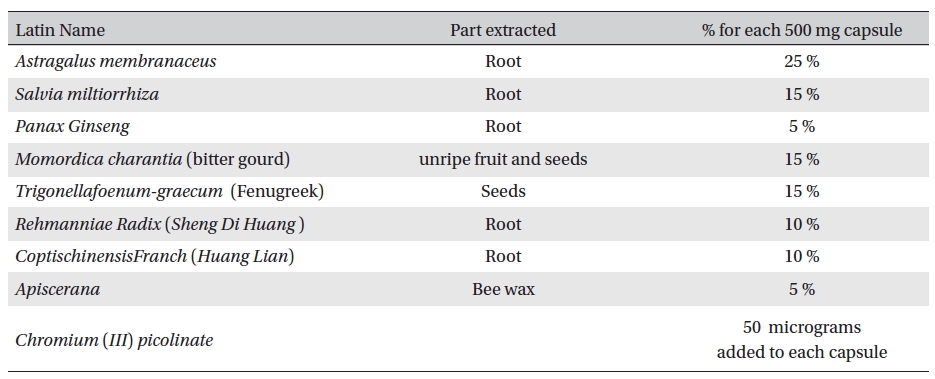
Ingredients of the botanical compound used in this study
The major constituents of Danshen include water-soluble phenolic acids and lipophilic Danshinones. Phenolic acids possesses antioxidant and anticoagulant activities, whereas Danshinones show antibacterial, antioxidant, and antineoplastic activities. [16] Danshinone enhanced the activity of insulin in Chinese hamster ovary cells and in adipocytes. Pathways through which Dihydrotanshinone- I might affect blood glucose were examined in cellular experiments. Inhibition of advanced glycation end products through alpha-glucosidase blockade by danshen was reported. [17-19]
Some studies show that huanglian has cardiovascular effect. Intravenous injection of berberine at 0.1 to 6.0 mg/kg, lowered blood pressure in anesthetized dogs, cats and rats. The mechanism of blood pressure reduction is dilation of the blood vessels and inhibition of secretion from adrenal glands. Berberine also has antiarrhythmic actions. [20-21] The flavonoids and antioxidant phenols concentrated in Propolis are powerful antioxidants and have been shown to be capable of scavenging free radicals which can extensively interfere with normal cell metabolism. Active free radicals, together with other factors, are considered to be responsible for cellular ageing and degradation in conditions such as cardiovascular diseases, arthritis, cancer, diabetes, and neurodegenerative diseases [22-25].
Chromium (Cr) is an essential element required for normal carbohydrate metabolism. A preliminary study found that treatment with corticosteroids caused increased loss of chromium in the urine [26]. Another preliminary study found that individuals with corticosteroid-induced diabetes could improve blood sugar control by taking chromium supplements [27]. Trivalent Cr, the form of Cr found in foods and nutrient supplements, is considered one of the least toxic nutrients. [28]
Sample no. 1 was a 500-mg capsule of Glyco-Persica. Its ingredients are shown in Table1.
Sample no. 2 was a 500-mg placebo capsule, filled with post-extracted washed fibers. Two capsules (1000 mg) were administered three times daily for 45 days.
The inclusion criteria were age 24-70, fasting blood sugar > 200 mg /dl, 2 h postprandial blood sugar > 200 mg/dl, history of diabetes type 2 of more than one year and treated with allopathic medications.
Exclusion criteria were pregnancy, severe heart failure, renal failure, liver failure and other complications. Patients with other primary diseases, such as diabetic ketoacidosis and who under taken corticosteroids or other medicines that affect blood sugar blood sugar were also excluded.
We used double blind random selection to divide the patients who had been treated between March 2007 and November 2009 and who were willing to participate in this trial; according to their blood glucose into treatment and a control groups. A balanced test among all factors that might affect the course of disease such as medication, Type, gender and age, was done to avoid comparability between each two groups. All participants took medicine every day for 45 days. All indices were measured before and after intake of food. Safety indicators included general physical examinations:
Detailed inquiries regarding participant’s mental condition, sleep routine, diet, urine and stool, body weight, blood pressure, and heart rhythm before the start of this study; complete blood count assessments: red blood cells, white blood cells and differentiation, and hemoglobin.
Routine stool tests were done and included routine urine analysis: pH, white blood cell count (WBC), sugar, and ketones.
Blood biochemical tests for serum total protein, albumin, alanine transaminase, aspartate transaminase, blood urea nitrogen, serum creatinine, blood glucose, total cholesterol, triglycerides, and high-density lipoproteins.
Chest x-rays, electrocardiograms, abdominal sonograms were taken before the test. Finally, the side effects of medications were observed.
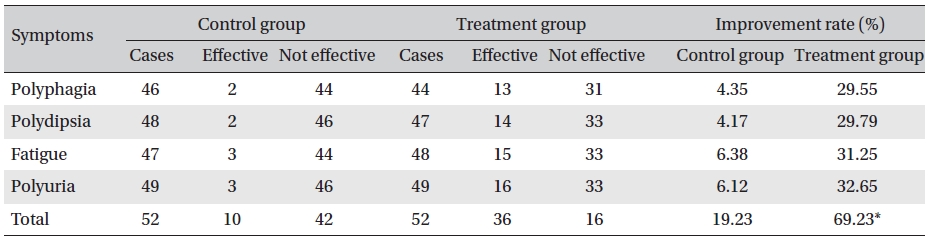
Detailed inquiries were made about the participant’s history, diet, medications, and activities. In all cases, we determined the chief complaint such as polydipsia, polyuria, polyphagia, fatigue, etc., before and after food, and we divided the symptoms into three categories according to severe as 3, medium as 2 and mild as 1. We also observed symptom improvement. (Improvement of more than one for each symptom was considered effective.)
Glucose tolerance tests were done before and 2 h after the consumption of 10 large dates or bread made from 100 gram of flour.
Urine sugar and ketones were measured. Morning urine was measured on an empty stomach, and according to positive rate, the results were divided into - , ±, +, ++, +++, ++++ groups corresponding to 0, 0.5, 1, 2, 3, and 4 points, respectively. Before and after food, we statistically analyzed the integral values.
Basic symptoms were considered to be reduced significantly in the treatment group when a significant difference in the fasting blood sugar or the 2-h post-prandial blood glucose existed between the treatment and the control groups or between the treatment group before and after treatment. A drop of ≥10 % compared to the pre-test blood sugar was considered a significant difference.
Results are expressed as means ± standard errors. Paired sample
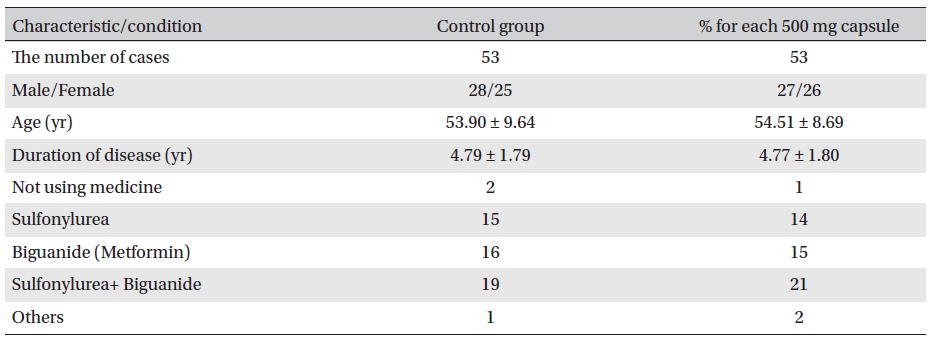
Double-blind observations were made. Capsule 1 was used for the treatment group, and capsule 2 for the placebo group. In general, the two groups were comparable(Table 2)
Clinical symptom scoring and clinical symptoms with changes are shown in (Table 3) and 4, respectively. Clinical symptoms were compared between the treatment group before and after treatment and between the treatment and the control groups after treatment, and the differences were statistically significant (
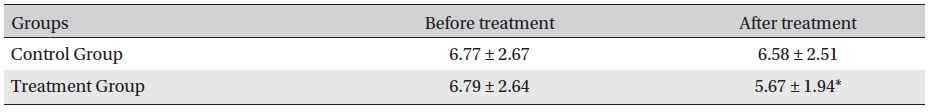
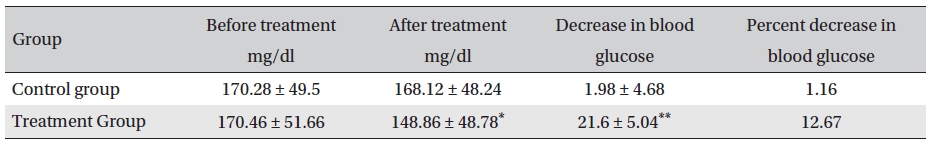
Before treatment, the fasting blood sugar levels in the two groups were not significantly different (P > 0.05); neither were the fasting blood sugar levels in the control group before and after treatment (P > 0.05). However, the fasting blood glucose level in the treatment group after treatment compared with that before treatment and with that of the control group after treatment was significantly different (P < 0.05). After treatment, the blood glucose levels in the treatment and control groups were lower by 21.6 mg/dl (12.67% reduction) and 1.98 mg/dl (1.16% reduction), respectively, the difference being statistically significant (P <0.05) (Table 5)
[Table. 2] General characteristics and conditions before treatment
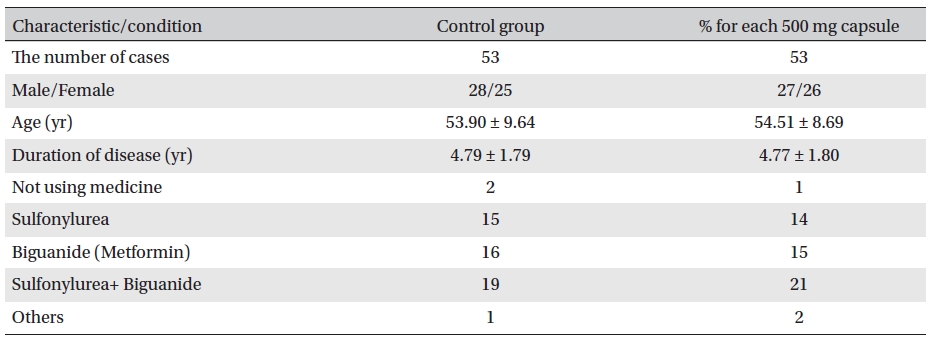
General characteristics and conditions before treatment
[Table. 3] Clinical symptom scoring (integral value, mean ± SD)
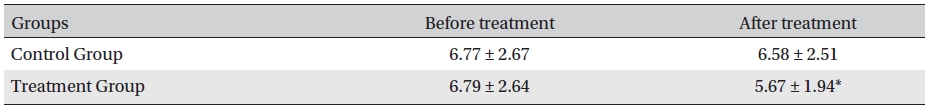
Clinical symptom scoring (integral value, mean ± SD)
[Table. 4] Changes in clinical symptoms
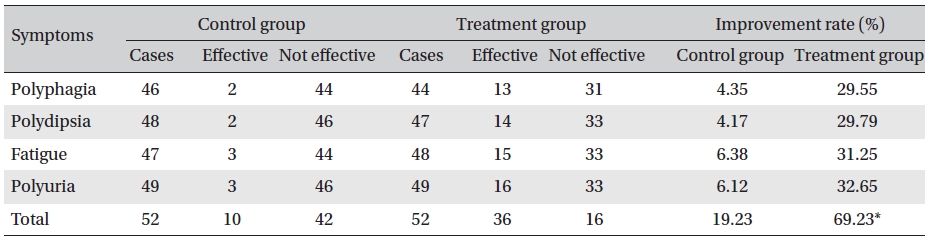
Changes in clinical symptoms
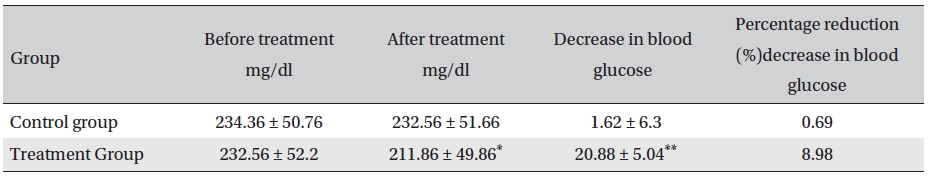
Before treatment, the post-prandial blood glucose levels in the two groups were not significantly different (
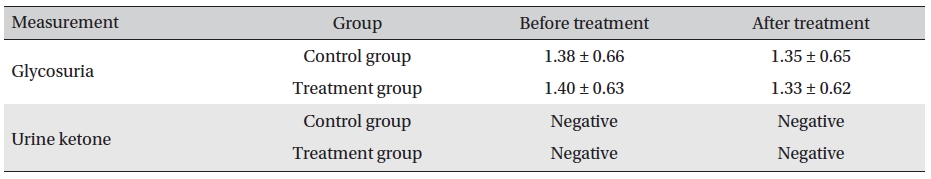
In both the two groups, the urine glucose levels after treatment were not significantly different. Also, in the treatment and the control groups, the levels before and after treatment were not significantly different (
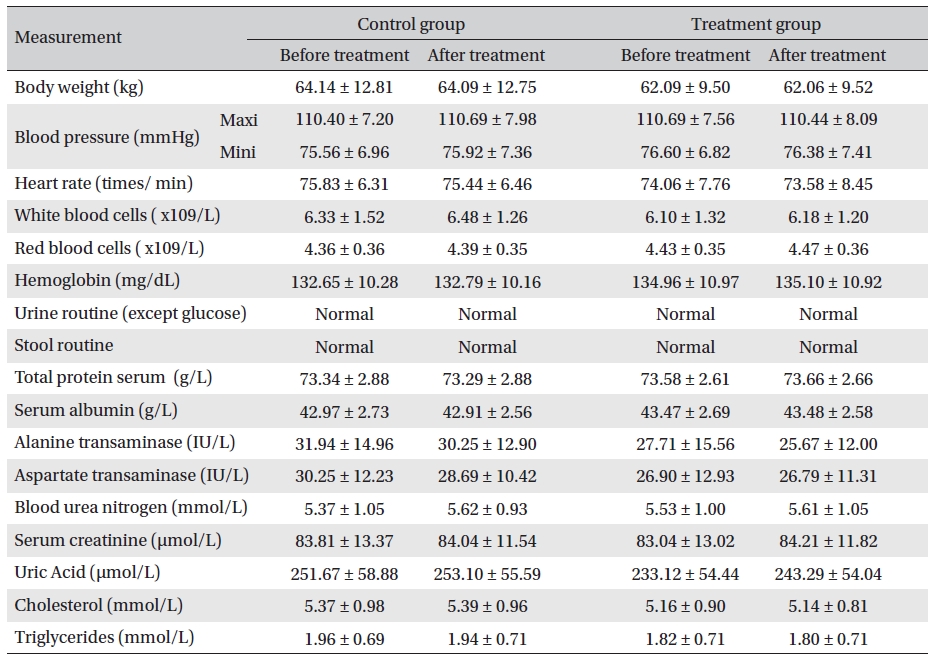
Body weight, blood pressure, heart rate, routine blood and stool/urine checkups (except urine glucose), and biochemical tests showed no apparent changes. No significant adverse reactions occurred during evaluations. The Glyco-Persica medication caused no obvious damage to the body (Table 8)
After 45 days of treatment, one case each in the groups was eliminated due to intermittent use the placebo and the herbal compound such that we could not determine a result. The final number of patients in each group was 52 (Table 9)
[Table. 5] Fasting blood sugar levels before and after treatment (mg/dl, mean ± SD)
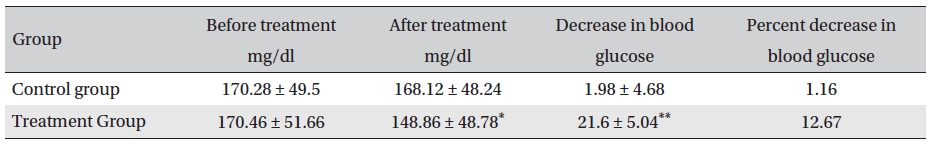
Fasting blood sugar levels before and after treatment (mg/dl, mean ± SD)
[Table. 6] Post-prandial blood glucose changes (mg/dl, Mean ± SD)
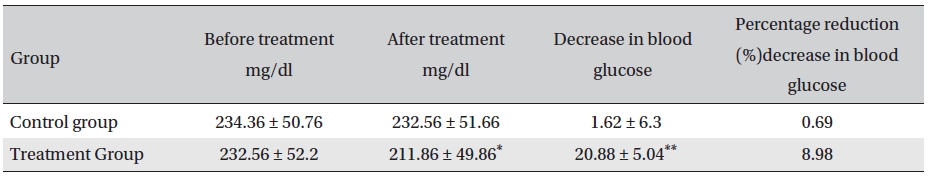
Post-prandial blood glucose changes (mg/dl, Mean ± SD)
[Table. 7] Evaluation of glycosuria and urine ketone before and after treatment (mean ± SD)
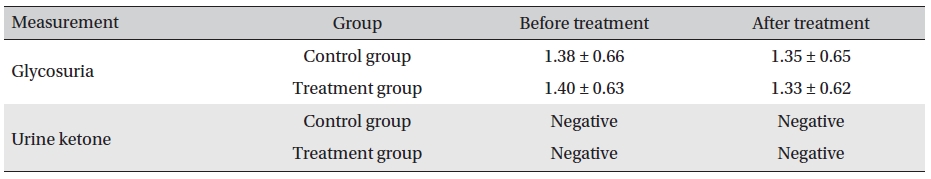
Evaluation of glycosuria and urine ketone before and after treatment (mean ± SD)
The results showed that Glyco-Persica significantly lessened the main clinical symptoms in diabetes type 2 patients (treatment group: 69.23%, control group: 19.23%). The fasting blood sugar in the treatment group after treatment was significantly different from that in the treatment group before treatment and from that in the control group after treatment (P < 0.05). In the treatment group, the reduction in the fasting sugar after treatment was significant compared to that in the control group (12.67% vs. 1.16%, P < 0.05). Also, the post-prandial sugar in the treatment group after treatment compared with that before treatment and with that in the control group after treatment was significantly different (8.98% vs. 0.69%, P < 0.05).
The results revealed that Glyco-Persica® had a significant hypoglycemic effect on the main clinical symptoms in diabetes type 2 patients, but body weight, blood pressure, heart rate, routine blood and stool/urine checkups, and biochemical tests showed no apparent changes. No significant adverse reactions were noted during evaluations.
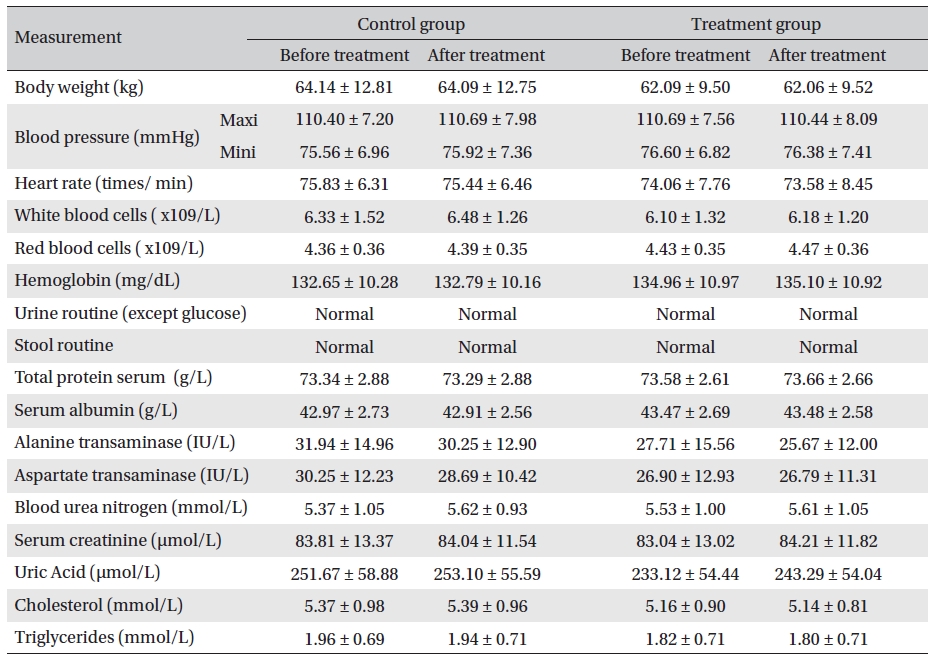
Analysis of body weight, blood pressure, heart rate, routine blood, stool/urine and blood biochemical index before and after treatment (mean ± SD)
[Table. 9] Trail screening rate

Trail screening rate