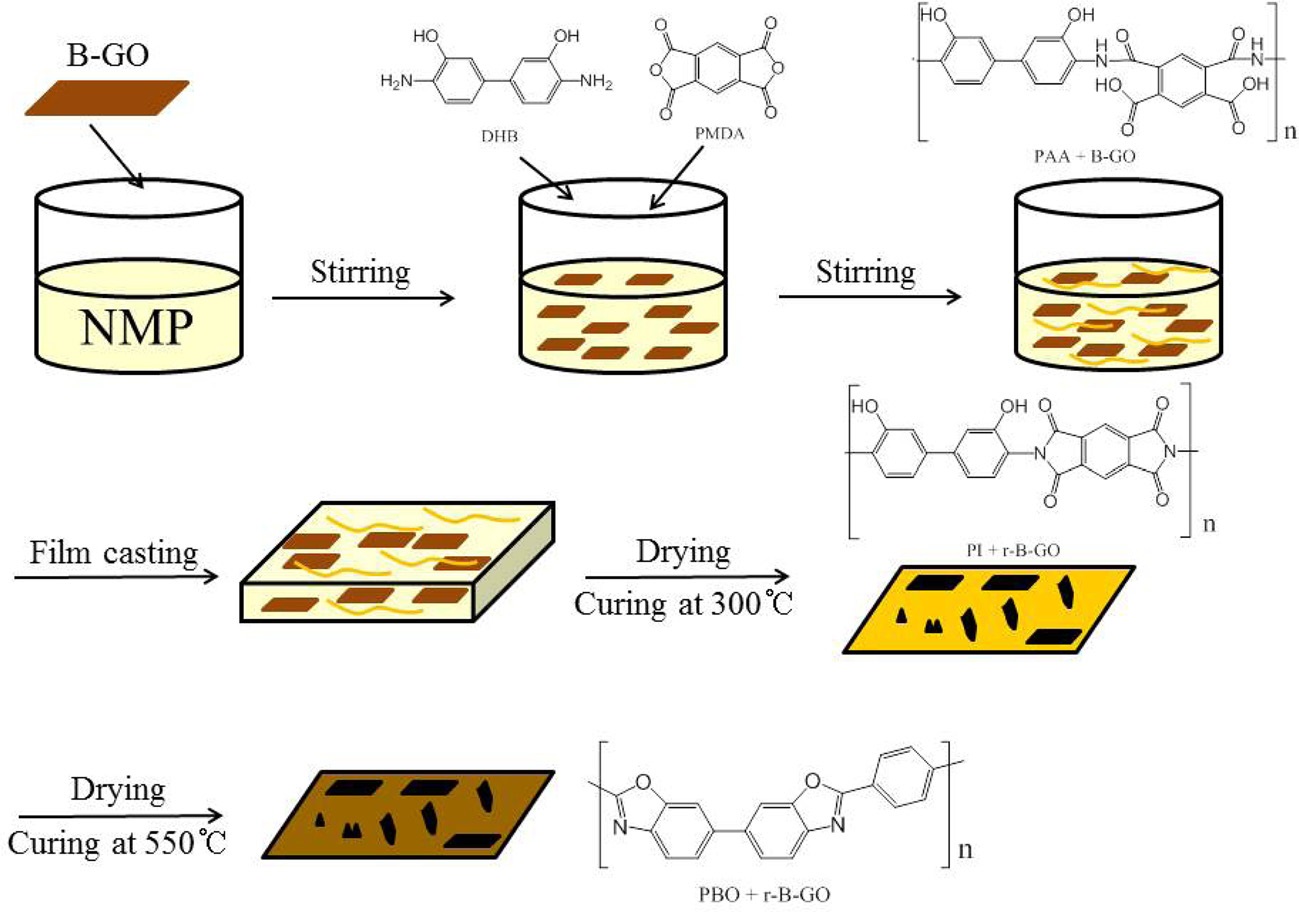
In this study, poly(amic acid) was prepared via a polycondensation reaction of 3,3’-dihydroxybenzidine and pyromellitic dianhydride in an N-methyl-2-pyrrolidone solution; reduced graphene oxide/polybenzoxazole (r-GO/PBO) composite films, which significantly increased the electrical conductivity, were successfully fabricated. GO was prepared from graphite using Brodie’s method. The GO was used as nanofillers for the preparation of r-GO/PBO composites through an in situ polymerization. The addition of 50 wt% GO led to a significant increase in the electrical conductivity of the composite films by more than sixteen orders of magnitude compared with that of pure PBO films as a result of the electrical percolation networks in the r-GO during the thermal treatment at various temperatures within the films.
Polybenzoxazoles (PBOs) are rod-like polymers with good mechanical properties, superior thermal stability, good chemical resistance, low dielectric constant, and good processability [1,2]. Therefore, they have been widely used in IC chips, interlayer dielectrics for multilayer electronic devices, and high-end electronic packaging materials, as well as a variety of photoelectric devices [3]. The improved mechanical and optoelectronic properties have been demonstrated in carbon nanomaterials including carbon nanotubes (CNTs) and graphene into the polymer matrix [4,5].
Graphene, which is a two-dimensional carbonaceous material of sp2-bonded carbon atoms, has attracted significant attention due to its extraordinary electric, thermal property, and high surface area [6,7]. As with CNTs, graphenes have limitations in composite applications, because they are very difficult to disperse in both organic and inorganic solvents due to their significant van der Waals force [8]. In contrast, graphene oxide (GO) prepared via the chemical oxidation of graphite can meet this demand because it can be dispersed effectively in water and organic solvents due to its functional groups including epoxides, hydroxyls, ketones, and carbonyl groups [9-11]. Moreover, GO, which is obtained from graphite, is cost effective compared with graphene and CNTs.
Recently, many researchers have developed composite materials using GO and reduced-GO (r-GO) as nanofillers for enhancing the mechanical and electrical properties using a small amount of GO (<10 wt%) [12,13]. However, few reports have focused on nanocomposites with a high GO content.
In this study, we report an effective method of preparing r-GO/PBO composite materials via
films were examined and scanning electron microscopy (SEM) was used to observe the morphology of the fractured surface of the films.
Graphite powders (99.9995% purity) were purchased from Alfar Aesar (USA). Fuming nitric acid (HNO3, 99.5%) and sodium chlorate (NaClO3) were purchased from Sigma-Aldrich (Korea). Hydrochloric acid (HCl, 35%) was obtained from Daejung Chemical (Korea). 3,3’-Dihydroxybenzidine (DHB) and pyromellitic dianhydride (PMDA) were obtained from TCI (Japan) and Lonza (China), respectively. N-methyl-2-pyrrolidone (NMP, 99.5%) was also purchased from Sigma-Aldrich (Korea).
GO was synthesized using the Brodie method. First, 1 g of natural graphite and 8.5 g of NaClO3 were mixed using a stirrer at room temperature. Then, 9 mL of fuming nitric acid was slowly poured into the mixture using a dropping funnel in an ice bath and stirred for 24 h at ambient temperature. An additional 5 mL of fuming nitric acid was added into the mixture. Next, the mixture was heated at 60℃ and stirred for 24 h. After that, the mixture was neutralized using 200 mL of deionized (DI) water and washed with 100 mL of 3 M HCl. After filtration, the neutralized mixture was dried in a vacuum oven at 50℃ for 24 h.
2.2. Synthesis of poly(amic acid) and its nanocomposite solutions
PMDA (0.500 g, 2.3 mmol) was added to a solution of DHB (0.496 g, 2.3 mm) in NMP (10 mL). An additional NMP solution was added until the solid content was adjusted to 15% by weight. The mixture was stirred at room temperature for 24 h in order to produce a viscous and homogeneous poly(amic acid) (PAA). Using the 1 wt% GO/PAA composite solution as an example, the detailed synthesis procedures were as follows: 0.010 g of B-GO was added to the NMP solution and the mixture was subjected to sonication and homogenization for 1 h. Then, 0.496 g of DHB was added to the GO solution. Next, 0.500 g of PMDA was added to the mixture. Finally, 1 wt% GO/PAA solution was obtained with the desired viscosity. A series of GO/PAA composite solutions with different GO loadings (1~50 wt%) were prepared using the same method.
2.3. Preparation of pure polyimide and its composite films
The conventional solvent casting method was used to fabricate the PAA and GO/PAA composite films. A horizontal bar was used to control the thicknesses of the films with a casting speed of 6 mm. Pure PAA and GO/PAA solutions with a thickness of approximately 750 μm were cast onto the commercial polyimide (PI) film. All cast films were dried at 90℃ for 2 h in a vacuum oven to remove the residual solvent. All films were heated under a nitrogen atmosphere for 30 min at 100℃, 150℃, 200℃, 300℃, 350℃, 450℃, and 550℃.
The chemical reaction was confirmed using a Fourier transform-infrared spectroscopy (FT-IR Spectrophotometer, Nicolet IS10, USA). The electrical conductivities were measured using a four-probe resistivity meter (FPP-RS8, Dasol Engineering, Korea) and a super megohmmeter (SM-8200, Hioki, Japan). The thermogravimetric analysis (TGA) was performed in order to investigate the stability and thermal conversion from PAA to PBO in a nitrogen atmosphere at a temperature increase of 10℃/min using a TGA Q50 (TA Instruments, USA). The analyses of the fractured cross-sections were observed using a field emission SEM (FE-SEM, FEI, USA).
GO as the filler in the synthesis of PAA was obtained using the Brodie method and then dispersed in the NMP solution using ultrasonication for a uniform dispersion. The GO/PAA films
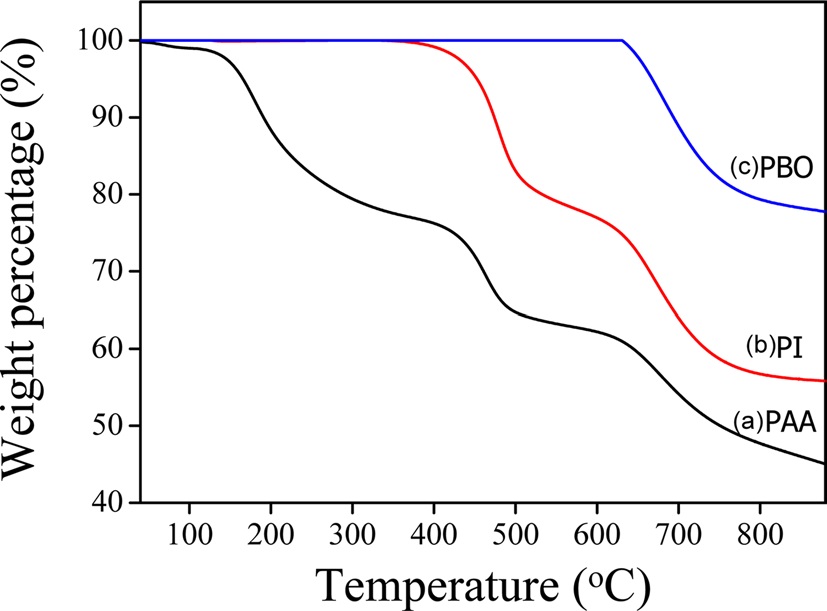
were prepared via casting onto the PI film using heat treatment in order to obtain the r-GO/PBO composite films as shown in Scheme 1. Fig. 1 presents the TGA thermograms of PAA before and after the thermal treatment. The first weight loss resulted from the thermal dehydration from PAA to PI.
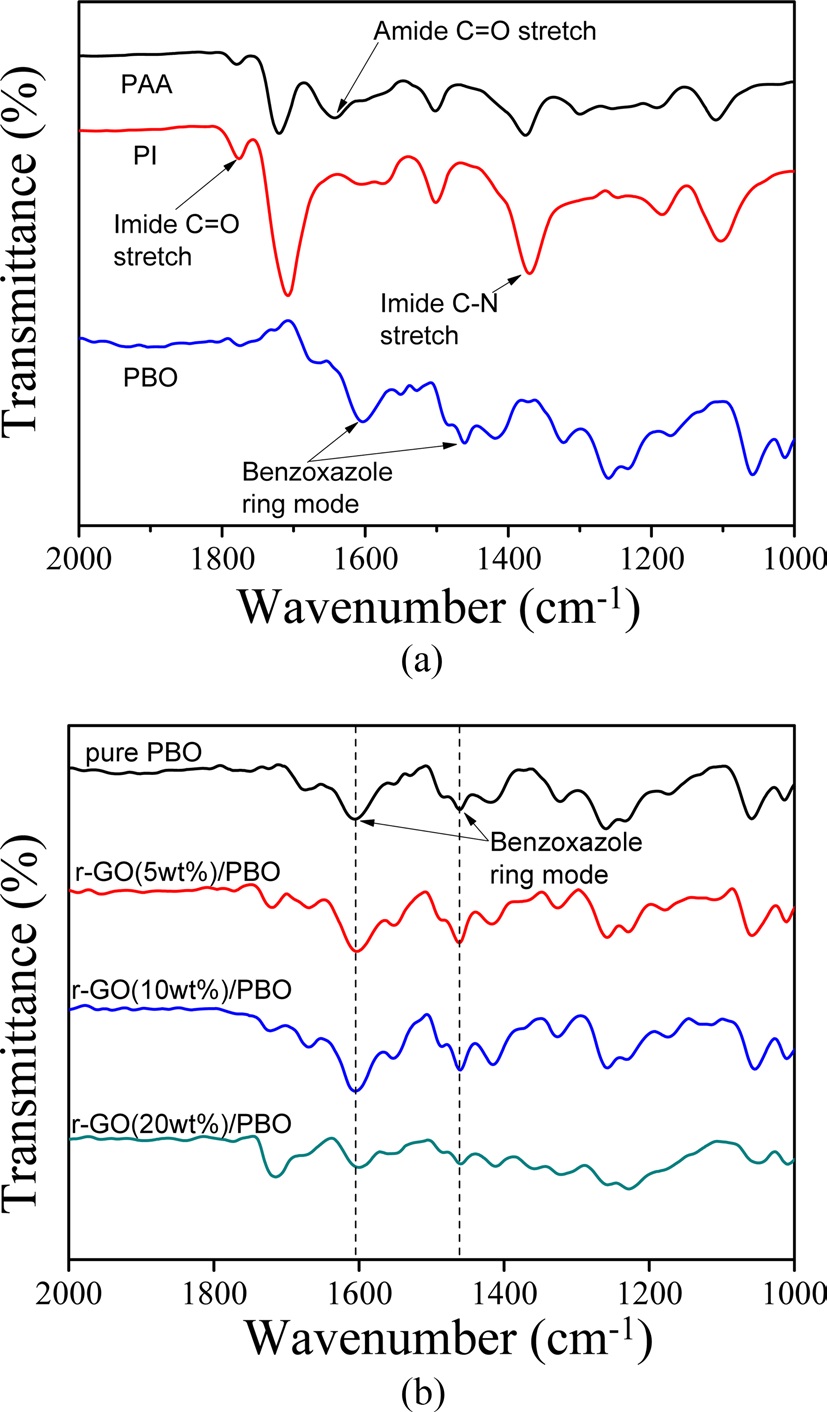
The second weight loss originating from the cyclocarboxylation (-CO2) was attributed to the thermal conversion from PI to PBO and appeared between 400-500℃. The FT-IR spectra of PAA, PI, PBO, and their composites are shown in Fig. 2. Prior to heating, the characteristic absorption peaks that originated from the C-NH stretching (amide II) and C=O in CONH (amide I) stretching bands were identified at 1649 and 1538 cm-1. After the thermal conversion, the absorptions peaks originating from the imide moiety at 1716 cm-1 (C=O symmetric stretching), 1370 cm-1 (C-N stretching), and 723 cm-1 (C=O bending of imide) were observed. Finally, the absorption peaks at 1600 and 1460 cm-1 were confirmed to form the benzoxazole ring [14]. The FTIR spectra of the r-GO/PBO composites at 1600 and 1460 cm-1 that result from the PBO unit were observed as shown in Fig. 2b. However, there was no significant difference between PBO and its composites. These results indicate that the r-GO/PBO composite films were successfully obtained. The fractured surfaces of the pure PBO and r-GO/PBO composite films were observed via SEM as shown in Fig. 3. As shown in Fig. 3a, the fractured surfaces of the pure PBO film were relatively smooth compared with the r-GO/PI composite containing 5 to 20 wt% of GO in Figs. 3b-d.
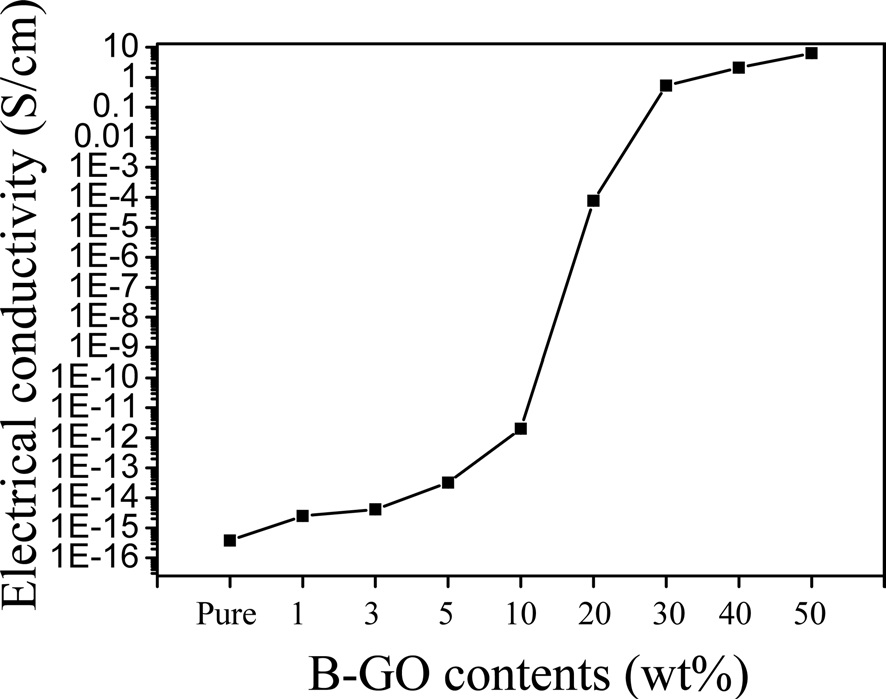
The electrical conductivities of the pure PBO and r-GO/PBO composite films were measured using the four-point probe unit. As shown in Fig. 4, the electrical conductivity of each sample was increased as the GO loading increased. The electrical conductivity of the pure PBO was 3.83 × 10-16 S/cm. However, the electrical conductivity of the r-GO/PBO composite containing 50 wt% GO was 6.31 S/cm, which is an increase of more than sixteen orders of magnitude more than that of pure PBO film. This occurred because the r-GO induced electrical percolation networks in the composites.
Using the Brodie method, GO was prepared from graphite as nanofillers for the preparation of r-GO/PI composites. The PBO was prepared via a polycondensation reaction followed by thermal curing at an elevated temperature and r-GO/PBO composite films, which significantly increased the electrical conductivity, were successfully fabricated. A FT-IR was performed in order to confirm the thermal conversion from PI to PBO and its composites with GO. The electrical conductivity of the r-GO/PBO composite film containing 50 wt% of GO was 6.31 S/cm, which is sixteen orders of magnitude more than that of pure PBO film due to the thermal conversion temperature above 550℃ used to produce PBO.