In India, forest based insect enterprises has never been linked up with any forest management activity, either as a forest conservation strategy or to reduce the poverty in forested area. This investigation indicated that when forest dependent people are associated with forest insect industry (FII) like forest sericulture, lac culture or apiculture; this income generating activity links livelihood with forest conservation, and generates a viable model of collaborative forest management (CFM). In this model different stakeholders work together as a coherent entity for unified goal of managing the forest for well-being of the poor people in fringe areas. Article summaries and evaluates the prospect of India specific forest insect industry, and discusses how and to what extent integration of FII could be a viable livelihood component in CFM to conserve the forest and insect biodiversity. We analysed a case study on forestbased rearing of tropical tasar silkworm rearing in Central India from CFM perspective. Arguments in this communication are intended to provide forest managers and policy-makers with necessary input to consider location specific FII in CFM mode to provide a continuous source of small income to forest dependent people to ensure long lasting success of their forest management endeavours.
India is a nation with over 330 million poor people1 (Thampi, 2012), a number that has barely declined over the last three decades of development. Forests in India support the household income of 833 million rural people (Census, 2011) in one way or other, but for about 200 million poor people2 who live in 1.73 lakh fringe villages3, forests are the only source for their livelihood (Nayak
On the other hand, recent data of International Monitory Fund (IMF) shows that India overtakes Japan to become third-largest economy in purchasing power parity (PPP) during 2011after US and China (Banerji and Shah, 2012), and very recently, a US intelligence community report released on 10th December 2012 predicted that by 2030, surging India along with decelerating China, would straddle global commerce and dominate the world economy amid the gradual decline of the west Rajghatta (2012). However, report also cautions that existing inequalities between urban and rural sector would further widen due to infrastructural and educational deficiencies (NIC, 2012). In India, youth bulges are very high and in next 15-20 years, the country will be challenged very high to find new jobs for its large youth population6.
Poverty in developing countries is the biggest problems and its alleviation is the greatest challenge for all governments. This study underlines the emerging role of forest insect industry (FII) in livelihood security of forest dependent people and evaluates the major impact of FII on forest ecology and management. We propose that location specific FII should be linked with CFM as a livelihood component to reduce poverty in forested area. Arguments in this communication are intended to provide forest managers and policy-makers with necessary inputs to consider FII as a viable commercial vehicle in CFM.
Comprehensive literature survey was carried out to observe the efficacy of forest insect industry in livelihood delivery of the forests in terms of improved household income and its impact on poverty reduction. Main findings ware considered to make an informed discussion about India specific viable FII. Results of Bhatia
>
Forest insect industry (FII) and its emerging role in poverty alleviation and forest management
In recent years, numerous actions for sustainable exploitation of forest resources have been undertaken (Muafor
Many forest insects are popular foods in different cultures all over the world, but only few are commercialized (de Beer and McDermott, 1996). Women and children mostly collect edible insects from forest (FAO, 2012) and their trades makes significant contribution in household economy of many forest dependent people in Sub-Saharan Africa (Vantomme
Apart from the contribution of forest insects in food security, investigations have indicated that they contribute significantly to livelihoods earnings in both rural and urban areas (Stack
In Central Africa, caterpillar of
Further, in Papua New Guinea (PNG), the Queen Alexandra’s birdwing butterfly (
The mopane worms (MWs), an edible larva of Saturniid moth,
In Java, Indonesia, larvae and pupae of Asian weaver ant,
Further, in northeast Thailand, around 3 000 poor families are engaged in the rearing of house crickets,
In addition, bamboo borer caterpillar,
>
India specific forest insect industry (FII) and their livelihood potential
In India, commercial forest insect and their product have been traditionally associated with many tribal communities as a part time livelihood activity. However, in India, forest insect industry (FII) has never been linked up with any large-scale forest management activity or as a forest conservation strategy to reduce poverty in forested area. Moreover, most of the forest managers have little knowledge about manipulation of forest vegetation or harvest practices to maximize or sustain the population of forest insect to derive their livelihood function. However, forest managers can choose FII as one of the strategic priorities to increase contribution of forest to improve household earnings of poor people in forested area and concomitantly foster its impact on forest management. Secondly, owing to inconsistent livelihood delivery of many traditional NTFPs in India, FII can be an additional livelihood option rather than sole reliance on NTFPs and third; commercialization of forest-based insect enterprises can create many employment opportunities for bulging youth population in rural India.
Among many forest insects, wild silkworms, forest honeybee, and lac insects have great promise in CFM. A brief account on how and to what extent these FIIs can be a beneficial adaptation in various geographical regions of the India are discussed below;
>
Integration of forest based sericulture (FBS) into CFM
Natural Silk, an insect protein fiber of silkworm cocoon marks class and excellence to the rich people but for the poor families who rear the silkworms and the reelers and weavers who finish it into eco-friendly textiles, silk stands for a natural source of livelihood. Commercial silk producing insects of order Lepidoptera are classified into mulberry and non-mulberry sector. In India, non-mulberry or wild silk is now called Vanya Silk that includes tropical tasar or Indian tasar silkworm,
Among all forest insect industry (FII) in India, forest-based sericulture (FBS) is the largest human-insect interactions in terms of associated insects and forest tree species, number of poor families linked with and the forest area that it relates. Silkworm larvae are reared or grown naturally on different forest trees in or outside the natural forest. Production process of FBS is divided into pre and post-cocoon sectors. Pre-cocoon sector is forest-based and on-farm activity dealing with protection and management of the forest host tree for silkworm rearing to produce cocoons for raw silk production; however, reeling, spinning (yarn production from cocoon), and weaving are the post-cocoon and off-farm activity.
In India, FBS can be an ideal livelihood avenue for forest dwellers due to its low gestation and high economic return feature (CSB, 2012) that can provide vibrancy to rural economy by satisfying the equity concern of tribal women in their society, because FBS is predominantly women oriented business. Efficiency of forest-based sericulture (FBS) can be enhanced by training the forest communities in skills necessary for scientific rearing of different silkworm species. State Forest Department (SFD) can link JFMCs7 with State Department of Sericulture (DOS) for transfer of technology (ToT) and other extension support; Central Silk Board8 (CSB) can be associated for area specific R &D, training support and supply of silkworm seed; Credible NGOs may be linked up to ensure fare marketing of cocoon or finished yarn on competitive prices through viable market linkages, because in Vanya silk sector, there is no competitive marketing and State operated marketing agencies are some time blamed for institutional corruptions. Therefore, SDF, DOS, CSB, and NGOs can work together as a coherent entity to promote an effective model of CFM for unified goal of managing the forest for well-being of the poor people.
>
Tropical tasar silkworm, Antheraea mylitta Drury
Forest rearing of
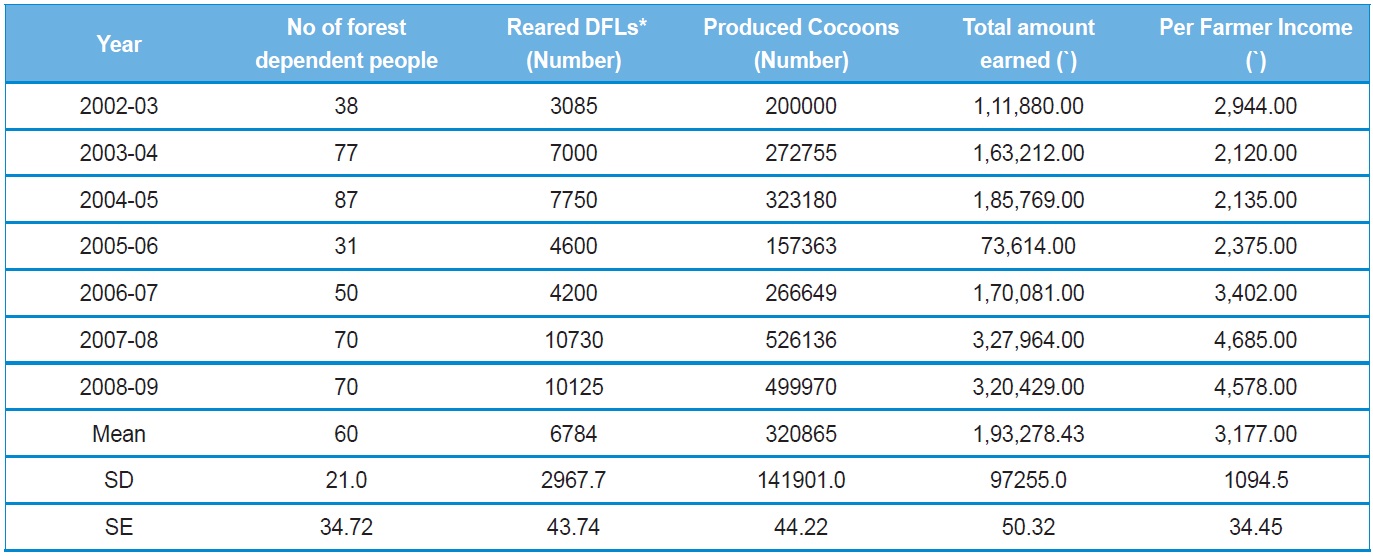
[Table 1.] Performance of tribal women's tasar silkworm rearing in Surguja, Chhattisgarh
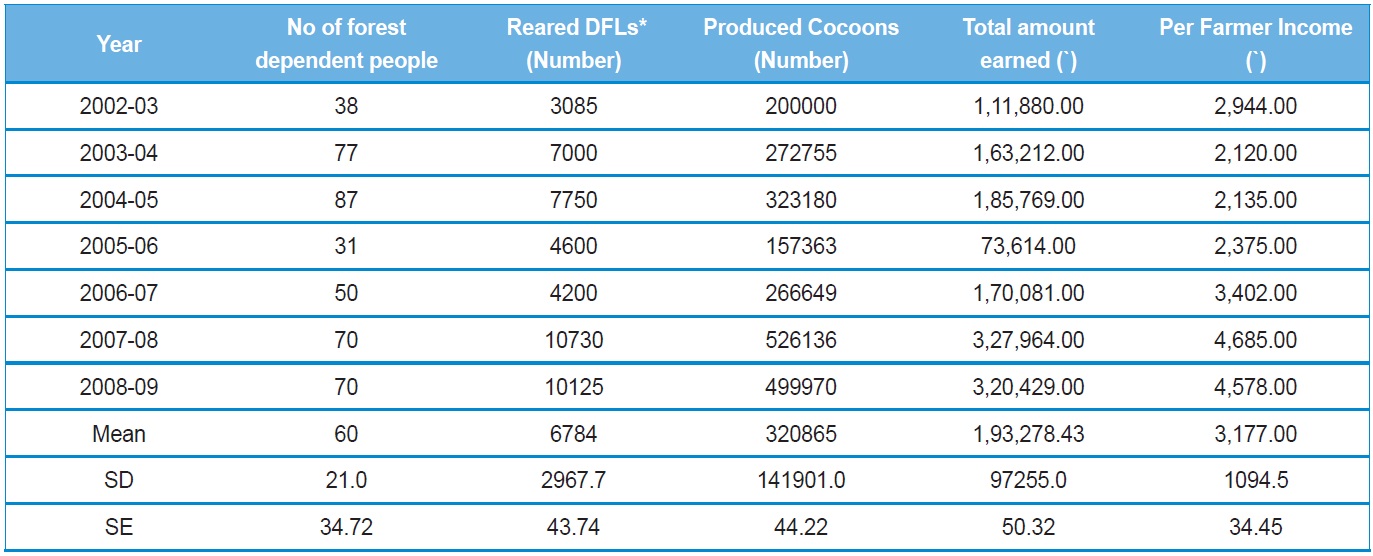
Performance of tribal women's tasar silkworm rearing in Surguja, Chhattisgarh
In nature,
In order to assess the impact of forest-based sericulture in livelihood improvement of the forest dependent people, and the role of this activity on forest management; Bhatia
In their study, Bhatia
>
Temperate oak tasar silkworm, Antheraea proyeli J.
Temperate oak tasar silkworms,
In north-eastern states of India, rearing of oak silkworm is the major forest insect industry that play a crucial role in rural economy (Unni
>
Muga silkworms, Antheraea assama Ww.
Literature survey indicates that
>
Eri silkworm, Samia cynthia Drury
Wild eri silkworm Samia cynthia D. syn.
Eri silkworm is the only wild silkworm that is domesticated indoor up to an altitude of 1525 m, where larval period lasts for 30-40 days. Host tree leaves are hung over the rearing tray and larvae climb and feed the leaves. Cocoon filament of eri silkworm is neither continuous nor uniform in thickness, which is hand reeled only. Tribals use produced silk indigenously for preparation of chaddars (wraps). Eri silk can be blended with cotton, wool, jute, or mulberry silk for jackets, suiting materials, furnishings, etc. Owing to polyphagous nature, hardy breed characteristics and indoor nature of rearing, eri silkworm raring can be integrated at large scale with CFM all over India.
>
Forest based sericulture (FBS) and Indian Forest (Conservation) Act, 1980
Recognizing the role of FBS in livelihood support to the poor families and contribution of this activity in forest conservation, Ministry of Environment and Forest, government of India issued guidelines for vanya silkworm rearing under Forest (Conservation) Act, 1980. According to this act, plantation of forest trees on which vanya silk worms could be reared without undertaking a monoculture plantation shall be traded as forestry activity, and no prior permission of the Central Government under Forests (Conservation) Act, 1980 is required. Provided that, such plantation do not involve any felling of the trees and while undertaking such plantations, at least three species are planted of which no single species covers more than 50% of the total planted area (MoEF, 2004).
>
Forest based sericulture and forest ecosystem
Forest based silk products are generated through ecological interactions between wild silkworms and their host trees. Insect eating green tree leaves in forest is an integral part of the forest ecosystem. Population build-up of insects are regulated by biotic, abiotic, density dependent, and density independent factors, and if natural regulatory mechanisms like parasites, predators, and pathogens are active, insects do not attain the status of pests and their population remains below to the economic injury level (EIL). There is no record that any saturniid has ever caused an outbreak in forest.
Functionally, annual primary production is an indicator of healthy forest ecosystem and forest productivity is the function of leaf area index (LAI). However, relationship between LAI and photosynthesis is not linear, because due to self-shading some leaves of the tree canopy receive so little light that their rate of respiration exceeds the rate of photosynthesis, which declines the function of net primary production against LAI. High leaf availability and corresponding increase in gross photosynthesis at high leaf area index is offset by increased respiration (Odum, 1975).
Silkworms are reared on forest host tree in small compact patches at outskirts of the forests and host trees are used once a year for 30-60 days. Host tree suffers a temporary photosynthetic loss, but the total production of forest stand does not likely to be affected, because foliage loss after silkworm feeding allows understory trees and other ground flora to get much light that compensates temporary photosynthetic loss of partially defoliated host tree. Secondly, after silkworms’ feeding, secondary foliage grows in 30-45 days and foliage losses are not only compensated, but the total LAI exceeds in comparison to the un-damaged trees. Third, the thickness of the secondary foliage is less than the primary foliage and has better photosynthetic efficiency in comparison to the older leaves, which were consumed by the silkworms. Fourth, increased longevity of newly grown leaves on silkworms’ fed trees extends the usual leaf fall period and this extended period of leaf on crown provides a positive photosynthetic gain to the utilized host tree. Therefore, as far as the foliage loss is concerned, new foliage offsets it and apparently, no loss takes place in annual primary production of the forest due to silkworm feeding. Furthermore, the roots of host trees grow more vigorously by utilizing increased nutrients contents due to enhanced microbial activity on larval fecal materials at the forest floor. Bhandari (2003) reported that forest silkworms are forest insects and have no adverse effect on growth and increment of the forest.
>
Integration of forest based apiculture (FBA) as a livelihood component in CFM
Honeybees are the important pollinators that ensure functioning of many ecosystems. According to FAO (2009), honeybees pollinate one third of all the plant products consumed by the humans. In US, Berenbaum (2007) estimated that bees pollinate over 90 crops for a value of more than US$15 billion in a year. All the four species of honeybee viz.,
Beekeeping is not a labor-intensive activity and can easily be accommodated in the daily routine of forest dependent people. Bee keeping has high earning potential where one can earn `1 70 000 from 100 bee colonies @ 20.11 Kg honey per hive and according to Sivaram (2012), if these colonies are put under diversification plan, this profit can be further increased up to `3 19 150 per year. Beeswax is twice costlier than honey; beecollected pollen, propolis, bee-venom, and royal jelly are several times costlier than beeswax; and all are in great market demand. Honey can be easily stored, sold, or consumed in the times of need.
Forests are known to provide organic honey, nectar, pollen, and propolis of high quality without any insecticidal residue that can be sold at premium prices in western markets through viable market linkages under CFM. In India, forest dependent people apply a very crude and destructive method of honey hunting to earn their livelihood and contribute 45.85% of the total honey production (85 000 MT) (Sivaram, 2012). They crush and squeeze the comb along with larvae and pollen grain that makes honey turbid and fetch low market price. Secondly, 40-50% forest bees discontinue their breeding cycles every year due to unscientific honey harvesting. Third, honey hunters use fire to facilitate honey collection that some time constitutes a source of forest fire.
FBK can be promoted through collective action of SFD, JMFCs, FRIs (Forest Research Institutes), and NBB (National Bee Board) in an adoptive CFM module, where forestry people and apiculture scientists can work together for safe honey extraction in forestry sector. FBK can increase the customary use of forest resources for equitable benefits sharing, forest conservation and poverty alleviation in forested area of the country. The important areas of interventions are capacity building for improved honey production, adoption of improved technologies for migratory bee keeping, nucleus stock maintenance, processing, and quality control of bee products. Beekeeping can be introduced as a special scheme in reforestation programmes by paying special attention to use native melliferous forest tree species to provide rich and varied source of nectar and pollen for forest honeybees. Secondly, bee-reserves can be established with exclusive access for beekeepers, as has already been done in United Republic of Tanzania (MNRT, 1998).\
>
Integration of forest based Lac culture (FLC) in CFM to alleviate poverty in tropical India
Lac is the only known commercial resin of animal origin secreted by female scale insect
Lac insects are reared on
Lac cultivation is simple that does not require any large investment and needs only part-time attention. The mean lac productivity varies from 1-10 kg per tree depending on host tree species and climatic conditions. Average net profit from one tree is `109 for
Transfer of technology (ToT) to deep tribal hamlet and timely supply of the quality brood lac are the important interventions to augment lac production in India, which can be ensured through institutional linkage of JFMCs with lac related R&D institute like Indian Lac Research Institute, Ranchi. Presently, only 15% of the available lac host trees are being utilized for lac cultivation. There is a vast potential for lac linked CFM in India.
>
Forest insect industry (FII) and insect biodiversity
Insects constitute more than fifty percent of the total biodiversity in tropical forests (Novotny
Diversity in the life style of an insect shows its capacity to adjust in different ecological conditions. For example, biodiversity of tasar silkworm reveals its potentiality and the genetic adaptability through interaction with environment to struggle and sustain in varying ecological niches through evolution of regional ecoraces. Overall, forest insect industry (FII) has a favorable impact on the conservation of insect and their habitat. For example, Holden (1991) observed a reduced frequency of bushfires in caterpillar harvesting areas of Zambia, where villagers sought to protect the sustainability of insect populations in natural forests. It is also reported that few edible insects enhance their habitat in specific ways, e.g. leaf-cutter ants in South America cultivate fungus gardens that convert cellulose into carbohydrates and in Africa, termites increase local plant species diversity, because some plants can only grow on termite mounds (De Foliart, 1997). Forest insect industry (FII) promotes conservation of insects in forest ecosystem by maintaining and encouraging the forest reserves by utilizing the forest buffer zone and it helps in maintaining heterogeneity of landscape by promoting concept of land sparing outside the forest reserves area (Samways, 2007).
>
Why and how to accommodate forest insect industry in CFM
Improved incentives encourage participation of local people in CFM
Forest insect industry (FII) has tremendous potential to contribute household income of forest dwellers in developing country. However, to link conservation with livelihoods, it requires an implementation model. Incentive linked forest management model acts as driving force for conservation and encourages the local communities to participate in forest management that provides them a direct stake in conservation efforts (Salafsky and Wollenberg, 2000). Such incentives become most attractive, when they are derived from sustainable use of forest biodiversity without threatening its resource base. Forest insect industry (FII) is a good option to realize these benefits, because it gives quick rewards and use renewable forest resources. Second, FII is technically simple and easily adoptable. Third, it encourages women participation and does not produce any chemical effluents in whole production process. Fourth, FII can serve a safety net function by creating employment to avoid poverty, and some of the poor people may grow out of poverty through continuous household earnings.
Institutional linkages encourage multi-governance mechanism under CFM
Community based conservation is conceived and implemented only at the local level and community institutions are only one layer in a multi-level world (Berkes
CFM encourages democratic way of functioning
Integrating the goals of forest conservation with livelihood requires capacity building that can be effectively addressed in CFM. Working together of different agencies addresses several socio-economical issues and decelerates tribal migration from forested area. Institutional linkages under CFM encourage democratic decision-making process and all the stakeholders have space and capacity to make them heard. Secondly, they communicate and transfer their knowledge and skills in multiple directions. Third, they jointly act to manage a conflict and fourth, there is a shared and intentional social learning and experimentation in forest management process, planning, and decision-making that clearly reflects a link to the desired future (Fisher
There is a good scope to integrate FII with CFM in India
In India, about 13 m ha forests are available with silkworm related forest flora as dense, open, and scrub forest (Srivastav and Thangavelu, 2005) that can be utilize to expand economic benefits of forest based sericulture (FBS) to a large number of forest dependent people.
JFM is the principal forest management strategy in India, but its potential for forest management and livelihood delivery is declining due to lack of institutional linkages and want of incentives to encourage community participation. JFM in India can be better utilized for location specific institutional linkages to realize commercial viability of FII. State Forest Department (SFD) may join other organizations beyond the forestry sector to reduce poverty in forested areas. Associative relation between different agencies with local community can better identify the hidden livelihood opportunity in the forest area and their viable integration can change the attitude of local communities by demonstrating the real potential of forests in terms of improved earnings. Such initiative by forest managers and policymakers will make forest-edge communities to aware of the precious value of forest insects in their life and the need to safeguard them; and by learning about the role of these commercial forest insects in forest ecosystems, people will better understand and appreciate the value of forests.
1. As per Census 2011, total population of India is 1210 million, equal to the combined population of USA, Brazil, Indonesia, Pakistan, Bangladesh, and Japan.
2. There is no census figure for forest dependent population in India. Different estimates put the figures from 200 million (See ICFRE, 2012) to 400 million (See MoEF, 2009).
3. As per the 2011 census, there are 6.41 lakh total villages in India.
4. As per the 18th Livestock Census 2007, livestock population of India is 530 million (See MoA, 2010). Thirty eight % of livestock depend on fodder derived from forest by direct grazing or by harvesting (See ICFRE, 2012).
5. In India, anyone monthly earning below to `672.8 (`22.42 per day) in the rural area and `859.6 (`28.35 per day) in the urban area is considered below the poverty line. Bihar (53.5 per cent), Chhattisgarh (48.7 per cent), Manipur (47.1 per cent), Jharkhand (39.1), Assam (37.9 per cent), and Uttar Pradesh (37.7 per cent) have high incidence of poverty. Among social groups in the rural areas, Scheduled Tribes suffer the highest level (47.4 per cent) of poverty, followed by Scheduled Castes (42.3 per cent) and Other Backward Castes (31.9 per cent) as against 33.8 per cent for all classes. In rural Bihar and Chhattisgarh, nearly two-third of the SCs and STs are poor where as in Manipur, Odisha and Uttar Pradesh it is more than 50 per cent. (See Planning Commission, 2012).
6. Over 35% of India population is below the age of 20 and 70% of India’s population is below the age of 35 years (See Census, 2011). By 2020, it is expected that 325 million people in India will reach working age, which will be the largest in the world.
7. JFMCs are village-level institutions of forest communities that are constituted democratically for the protection and development of forests and sharing of the benefits arising out of the managed forests, including NTFPs.
8. Central Silk Board (CSB) is a statutory and autonomous body under the administrative control of Ministry of Textiles, Govt. of India. CSB is an apex body, with responsibility of overall development of silk industry and sericulture in India.