ZnO(산화아연)와 rGO(환원 흑연산화물, reduced graphite oxide)로 구성된 복합체를 제조하여 중저온 영역(300-500 ℃)에 서 H2S(황화수소) 흡착실험을 수행하였다. rGO에 붙어있는 수산화기, 에폭시기, 그리고 카르복실기와 같은 산소를 포함하 는 관능기들이 H2S흡착에 미치는 영향을 조사하기 위해서 다양한 특성분석(TGA, XRD, FT-IR, SEM, 그리고 XPS)을 실시 하였다. GO(흑연산화물, graphite oxide)를 rGO로 환원시키기 위해서 마이크로파 조사법을 사용하였다. 마이크로파 조사 법에 의한 환원공정에서는 온화한 환원분위기를 조성하여 rGO 표면에 상당량의 산소 관능기들이 남아있는 것을 확인하였 다. 이러한 관능기들은 나노 크기의 ZnO가 2D rGO 표면에 균일하게 부착되도록 유도하여 고온 영역에서도 ZnO의 응집 및 소결이 일어나는 것을 방지하는 효과가 있다. 이로 인해 ZnO/rGO 복합체는 순수한 ZnO와 비교하여 3.5배 정도의 흡착량 을 보여주었다.
Hydrogen sulfide (H2S) is the most common sulfur component found in natural gas. It is an undesirable component in most industrial applications and is deleterious to the environment. H2S is known to be a major contributor to acid rain when oxidized in the atmosphere to SO2. In industrial processes, sulfur impurities rapidly deactivate or poison catalysts, which are widely used in the chemical or petrochemical industries[1]. Therefore, the removal of toxic gases (SO2, H2S etc.) has become a critical issue. Since the 19th century, various approaches to remove H2S, such as sorption, catalysis or condensation, have been applied[2]. Among those approaches, different adsorbents, such as activated carbon, zeolites[3,4], modified alumina[5] or metal oxides[6,7], have been investigated for H2S removal process. Historically, the high temperature range (500-800 ℃) H2S sorbents were extensively developed for hot-gas desulfurization (HGD)[2]. Zinc oxide (ZnO) is considered as a very effective sorbent for removal of H2S from hot gas streams, from a thermodynamic point of view, with the formation of zinc sulfide (ZnS)[8] (see Eq. (1)). An important drawback when using ZnO for hot-gas H2S removal process is its thermal instability to volatile metallic zinc[9]. However, for lower temperature applications, thermal stability is no longer an issue and ZnO can be converted to ZnS at ambient condition[10].
Graphene (2 dimensional, mono-atomic thick sp2-carbon structure) has recently received increasing attention as a material of interest due to its high electronic conductivity, large surface area and high mechanical strength[11,12]. Because of those benefits, most of graphene-based material studies focused on the electrochemistry field, such as battery[13,14] or super-capacitors[12,15]. More recently, graphite oxide (GO) with metal oxide composites have been extensively studied as adsorbents[16-18]. Graphite oxide-based or graphene-based materials are known to be useful for water purification, toxic gas removal and ammonia adsorption applications[19-21]. Graphite oxide which possesses oxygen functional groups attached on both sides of the surface received attention due to its ability to modify the physical properties and surface chemistry to enhance the interactions with target molecules[22]. The presence of oxygen groups on the surface of GO makes (or anchors) bonds with active metal oxides. Therefore, those oxygen functional groups are able to modify the availability of active sites on the surface of adsorbents depending on the dispersion of those active metal oxides and their chemical heterogeneity with GO[17]. When considering metal oxides for gassolid adsorption, one of the major issues is to avoid aggregation of nano-sized particles with temperature. Indeed, particle aggregation leads to losses in active surface area, with consequences of lower utilization efficiency of the adsorbent. Based on the above, the objective of the present study is to evaluate zinc oxide with reduced graphite oxide (rGO) composites as adsorbents of H2S at mid-high temperature. The morphology and surface chemistry changes from those composites are expected to affect the H2S removal efficiency.
2.1. Synthesis of graphite oxide
A modified graphite oxide (GO) synthesis procedure based on our previous work[23], was used in this study. Graphite oxide was synthesized usinga mixture of 360 mL of sulfuric acid (Sigma- Aldrich, ACS reagent, 95.0-98.0%) and 40 mL of phosphoric acid (Sigma-Aldrich, ACS reagent, ≥85 wt% in H2O), and 3.0 g of graphite powder (Sigma-Aldrich, <45 μm, ≥99.99%). This mixture was placed in an ice bath and when the temperature reached below 5 ℃, 18.0 g of KMnO4 (Samchun Chemical, 99.3 %) was added drop-wise. The mixture was stirred for 1 h and then transferred to a heating mantle to provide isothermal conditions at 50 ℃. The oxidation process was conducted for 18 h. The system was then cooled to room temperature naturally, and then placed in an ice bath again. 400 mL of de-ionized water and 15 mL of 30% H2O2 (OCI Company Ltd, 30 wt% in H2O) were added gradually. The mixture turned bright yellow and generated copious bubbles. The mixture was stirred for 1 h and then centrifuged at 3,500 rpm for 3 min. The remaining solid paste was washed with a mixture of 100 mL of de-ionized water and 100 mL of 30% HCl (Sigma-Aldrich, ACS reagent, 37%) twice. The product was then rinsed twice again with 200 mL of de-ionized water. After the washing steps, the paste was freezeand vacuum-dried overnight.
2.2. Synthesis of nano-zinc oxide (ZnO)/reduced graphite oxide (rGO) composite
400 mg of GO was dissolved in 200 mL of ethylene glycol (Sigma-Aldrich, Reagent Plus®, ≥99%) and then underwent ultra-sonication for 30 min. 100 mL of 0.1 M aqueous NaOH (Sigma-Aldrich, ACS reagent, ≥97.0%) solution was added, and the mixture was sonicated for an additional 30 min. Then, 100 mL of 0.07 M aqueous zinc acetate (Sigma-Aldrich, ACS reagent, ≥98%) solution was added into the mixture drop-wise (2.0 mL/min) for 50 min. 300 μL of hydrazine solution (Sigma-Aldrich, 35 wt% in H2O) was added before the reduction process. The zinc acetate/GO (ZnAc/GO) mixture was reduced by microwave irradiation for 3 min (1 min irradiation with 1 min break, 3 times). This reduction process produces ZnO/rGO composite. After cooling down, the ZnO/rGO mixture was filtered and washed with de-ionized water three times until the pH reached around 7.0. Finally, the paste was freeze- and vacuum-dried overnight. For reference, ZnO itself powder was also prepared.
2.3. H2S adsorption breakthrough tests
Dynamic breakthrough tests were conducted at moderate temperature (300 ℃). 0.5 cm3 of the adsorbents diluted with 1.0 cm3 of Al2O3 (Sigma-Aldrich, ~150 mesh) for a total of 1.5 cm3 of bed were packed into a quartz tube (internal diameter 10 mm). The mass of the adsorbents were measured around 0.158 g for ZnO/rGO and 0.355 g for ZnO. In a typical test, a flow of H2S (10.7 mL/min, 3.01 vol% of H2S balanced with N2) was mixed with 186.3 mL/min of N2 gas before passing through the adsorbent bed. The initial H2S concentration was 1,638 ppm with a total flow rate of 197.0 mL/min. The product stream was further diluted with 1800.0 mL of N2 before injection to the H2S analyzer due to the limitation of the H2S analyzer (Fluorescence H2S Analyzer, Model 101E, Teledyne). The experiments were carried out until the output H2S concentration reached ~10 ppm. The effects of CO2 and H2 on the H2S adsorption capacity of the sorbents were examined. 2.8 vol% of CO2 or H2 gases (5.0 mL/min) were mixed with the H2S/N2 stream (total flow rate of 197.0 mL/min) and passed through the adsorbent bed. In order to quantify the reactivity of ZnO with H2S, sorbent utilizations were calculated as follows:
where t is the experimental breakthrough time (min/g of ZnO), and t* is the theoretical breakthrough time (min/g of ZnO), which can be obtained from Eq. (3).
where VH2S is 0.32 mL/min here. From Eq. (3), the theoretical breakthrough time (t*) is calculated as 890 min/g ZnO. The experimental breakthrough time is determined when the outlet H2S concentration reaches 1ppm.
X-ray diffraction (XRD, Rigaku, 40 kV/100 mA of X-Ray, step size: 0.02°) was used for adsorbent characterization; 5-60° of 2
3.1. Characterizations of adsorbents
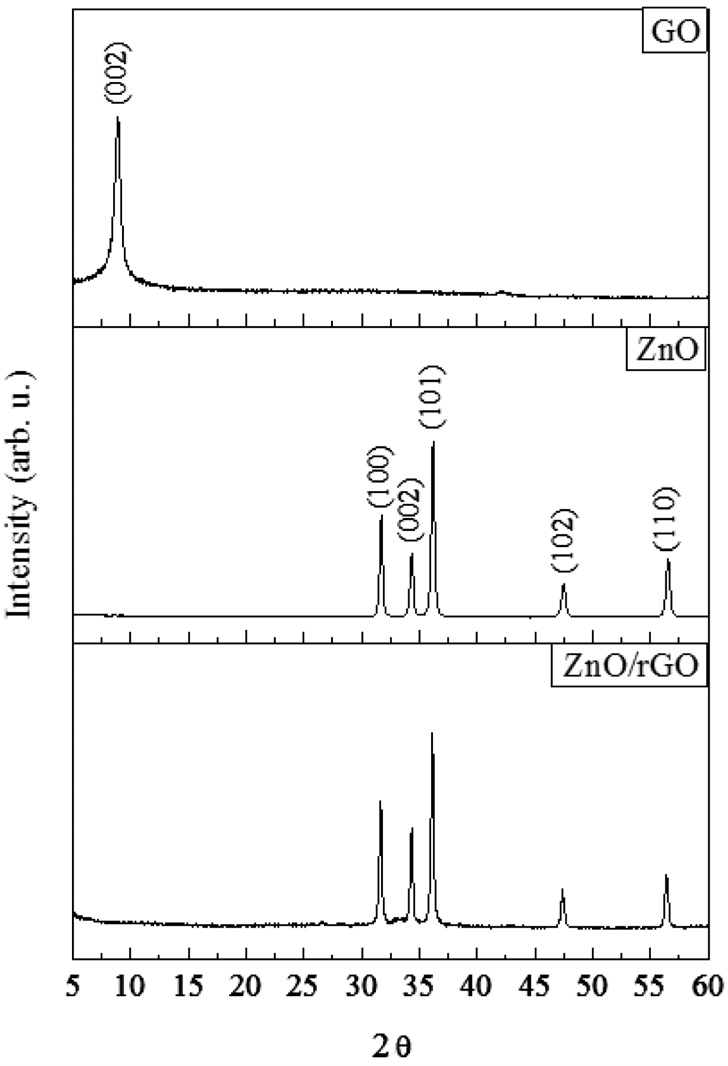
Graphite oxide (GO) is the product of the oxidation of graphite powder. It has been widely known that the 2θ characteristic peak of the GO is in range of 9-10°[24]. For ZnO/rGO composite in this study, the 2θ was located at 9.7° which is corresponding to the interlayer spacing of 9.2 A (Figure 1). It confirms that the interlayer spacing had been increased since it is widely reported that the typical interlayer spacing for graphite is 3.4 A [25,26]. From previous studies proposed that various hydrophilic oxygenated functional groups such as hydroxyl, carboxyl and epoxy are located between the graphene layers[27,13]. The characteristic
peaks for zinc oxide (ZnO) were shown at 31.6, 34.4, 36.1, 47.7 and 56.4° and those values are matched with JPCDS 36-1451. For this study, the ZnO/rGO had been prepared. After the microwave- assisted irradiation, the characteristic peak of GO had been collapsed; but ZnO peaks were remained. It implies that the oxygen containing functions groups were removed and graphene layers were detached each other; and turned to 2D layer substrate.
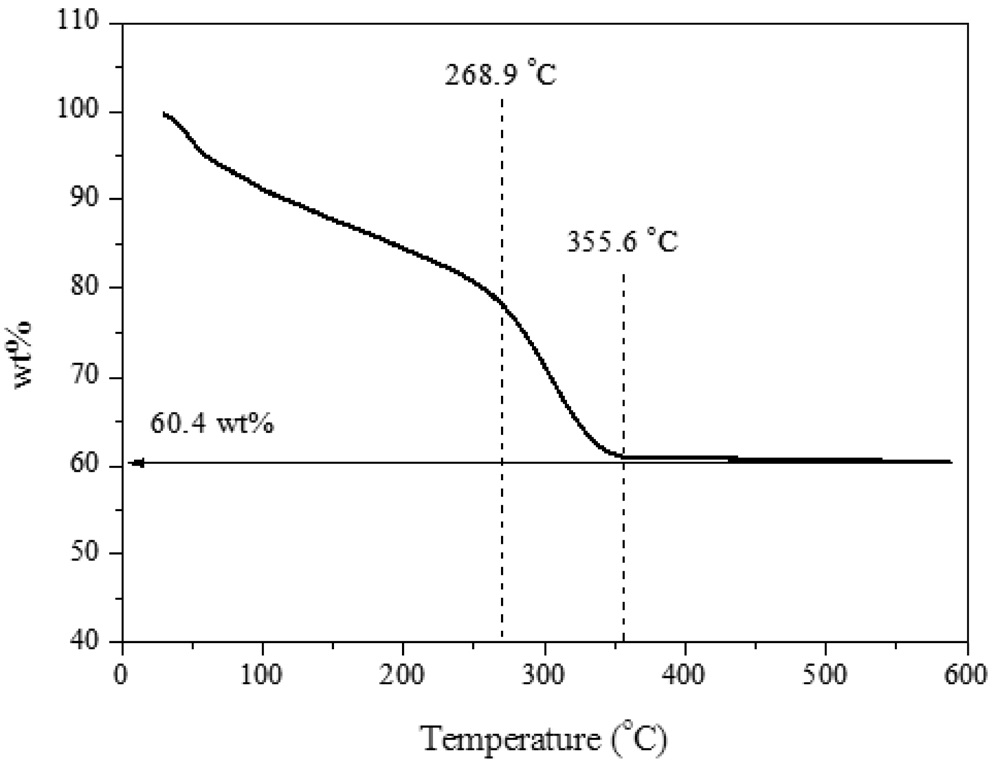
In order to examine the quantity of H2S adsorbed on ZnO, thermogravimetric analyzer (TGA) test was conducted (Figure 2). Two main phase changes were observed at 268.9 and 355.6 ℃. It could expect that at 268.9 ℃, the oxygen functional groups were destroyed; at 355.6 ℃, all remained carbon elements were
combusted. Then only ZnO had been remained; and no further weight decrease had been observed. It was estimated that 60.4 wt% was associated with ZnO. It could be concluded that the ZnO/rGO composite possesses 60.4 wt% of ZnO and rest of them should be considered as rGO component.
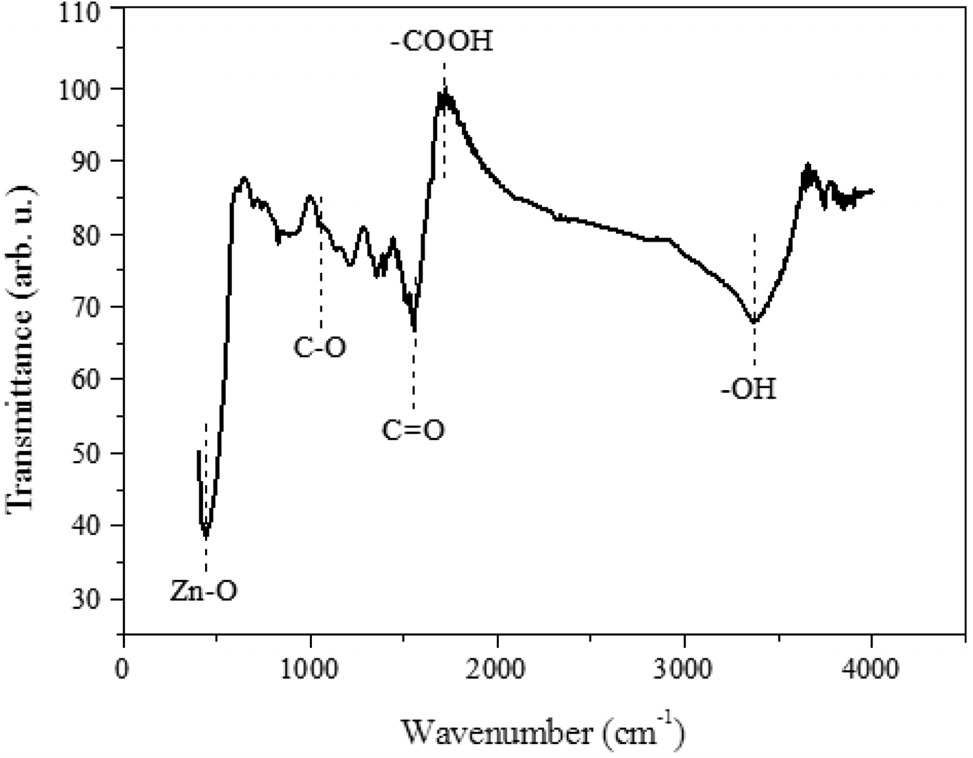
As mentioned above, the 3D GO had been converted to 2D rGO plane by the microwave irradiation while ZnO particles were loaded onto the rGO surface. From FT-IR (Figure 3), it was found that the oxygen functional groups were still remained on the rGO surface. From previous studies, those oxygen functional groups are acting as anchor sites to hold metal ions and promote the metal oxide dispersion[26]. A strong and broad absorption peak is located at 3,373 cm-1 which was assigned to the stretching vibrations of O-H. It implies that the rGO surface contains abundant O-H bonds; and those hydroxyl groups could act as anchor sites to hold ZnO particles. In addition, absorbance peaks at 1,720 cm-1 for carboxyl, 1,569 cm-1 for carbonyl and 1,052 cm-1 for epoxy.
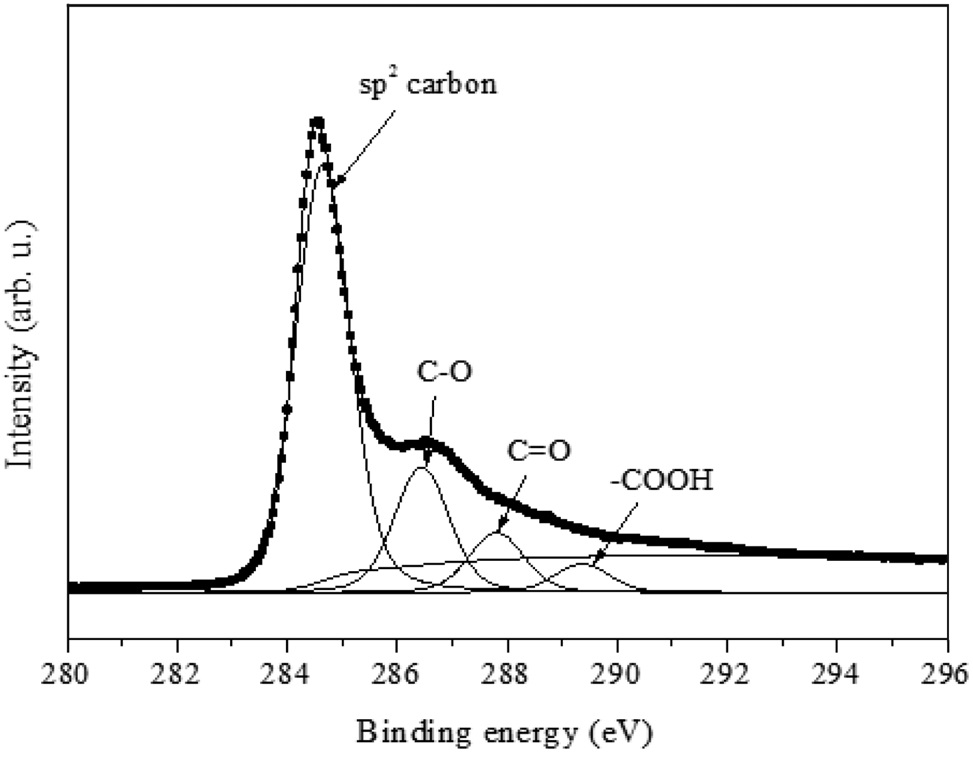
From FT-IR, the oxygen functional groups are identified. In order to get quantitative information, C1s XPS analysis (Figure 4) was conducted. Specifically, the binding energies of 286.5, 287.8 and 289.4 eV could be identified as epoxy (C-O), carbonyl (C = O) and carboxyl (-COOH), respectively; and 284.6 eV represents carbon-carbon sp2 configuration (Figure 4). By comparing the area ratio of the spectra (carbon-carbon/carbon-oxygen), the quantitative information of oxygen functional groups could be achieved. From previous study[23], the degree of oxygen portion for graphite oxide was 2.69 and 0.15 for graphene. For ZnO/rGO composite, the carbon to oxygen ratio was calculated as 0.39. It indicates that the amount of oxygen in the ZnO/rGO composite is less than GO but larger than graphene. It suggests that the microwave reduction method decreases the oxygen content from GO, but rGO surface contains oxygen functional groups even after the reduction process.
The direct support for the formation of nano-sized ZnO deposited onto the rGO surface could be achieved from SEM analysis (Figure 5). The average ZnO particle size was calculated as around 150 nm. The shape of ZnO was hexagonal; and it could be supported from the XRD characteristic peaks. The 2D rGO planes help dispersing ZnO particles which could increase the active surface area of ZnO. In addition, the ZnO particles were dispersed between the rGO planes since the metal salt (Zn2+) ions were well allocated between the rGO surfaces during the synthesis process[27].
As discussed above, the microwave irradiation process provides a mild reduction environment to GO since oxygen functional groups are still remained on the rGO surface. This was confirmed from the FT-IR and XPS analysis. Due to the oxygen functional groups, the metal salt ions (i.e. Zn2+) were anchored with the hydroxyl groups on rGO surface. Then it promotes the dispersion of the metal oxide (i.e. ZnO) through the rGO substrate. Those phenomena lead to increase the active ZnO surface for H2S adsorption.
3.2. H2S adsorption breakthrough tests
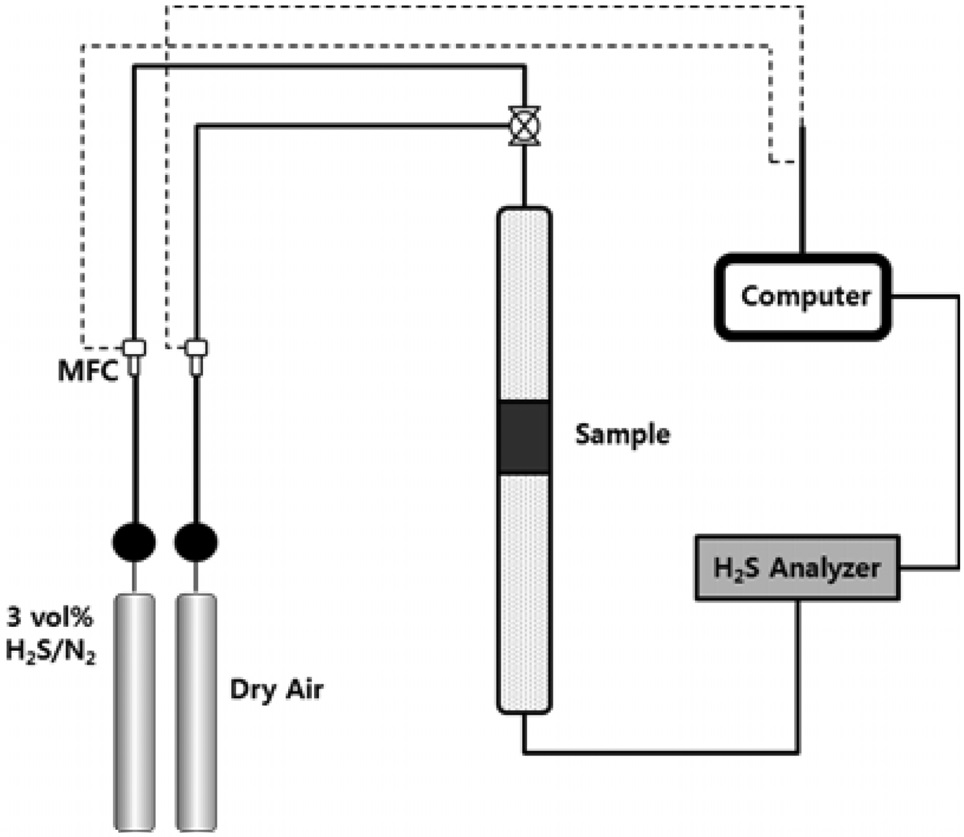
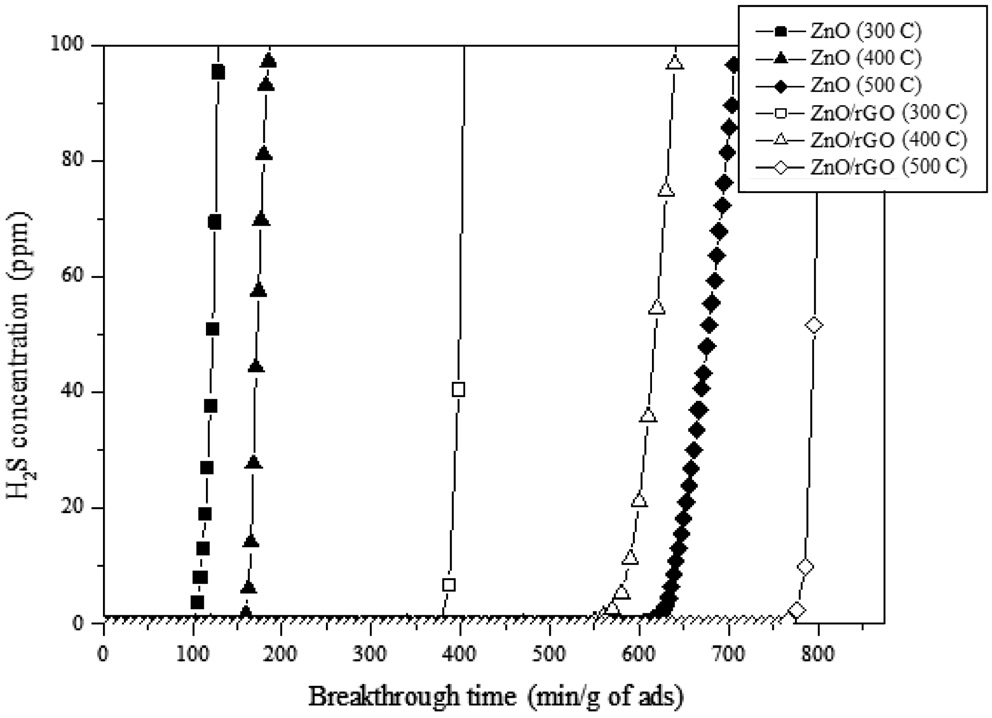
The schematic diagram of the H2S adsorption test is presented in Figure 6. 3.01 vol% of H2S (balanced with N2) was flowed through the adsorption bed. The reaction temperature was set to 300-500 ℃. From our previous study (data now shown), the graphene-based materials (i.e. GO, rGO and graphene) did not adsorb any H2S molecules due to the lack of active sites. Therefore, the H2S adsorption capacity shown in Figure 7 indicates that the adsorption capacity comes from ZnO. The average particle sizes of pure ZnO and ZnO in the ZnO/rGO composite were measured as 110 and 95 nm, respectively. Due to the thermodynamic energy, the H2S adsorption capacity for ZnO increased as the reaction temperature increased. At 300 ℃, the pure ZnO particle showed 105.4 min breakthrough time; at 400 ℃, 156.7 min and at 500 ℃, 598.3 min (per gram of adsorbent). However,
when the nano-sized ZnO particles were dispersed on the 2D rGO surface, the H2S adsorption capacities at various temperatures had been increased dramatically (i.e. at 300 ℃, 387.4 min, at 400 ℃, 549.9 min and at 500 ℃, 766.1 min/gram of adsorbent). It can be noticed that the ZnO/rGO composite showed the benefits at lower temperature since the ZnO/rGO composite showed about 3.7 times higher adsorption capacity than pure ZnO at 300 ℃. At higher temperature (400 ℃), the ZnO/rGO composite also showed about 3.5 times higher performance than that of pure ZnO. It indicates that even at low temperatures (i.e. 300 to 400 ℃) the well dispersed active ZnO particles exhibit the maximum ZnO utilization efficiency. From previous study, it was confirmed that the rGO substrate prevents the aggregation effect of the nano-sized ZnO at 300 ℃[26]. Those benefits preventing the aggregation of ZnO by using 2D rGO surface provide higher active surface area for H2S molecules which leads the higher H2S adsorption capacity. However, when high thermo energy had been provided (i.e. 500 ℃) to ZnO, the H2S breakthrough time had been jumped to 598.3 min per gram of adsorbent. The breakthrough time for the ZnO/rGO composite was 766.1 min/gram of adsorbent (about 1.3 times higher than that of pure ZnO). Those phenomena represent that the nano-sized ZnO particles deposited onto the rGO surface showed the benefits at relatively lower temperatures (i.e. 300 to 400 ℃) than higher temperature (500 ℃).
It has been studied that the 2D reduced graphite oxide (rGO) substrate plays a critical role in order to disperse the nano-sized (avg. of 95 nm) zinc oxide (ZnO) at mid to high temperature ranges (300 to 500 ℃). Due to the oxygen containing functional groups, such as hydroxyl, epoxy and carboxyl groups, the metal ions are able to be anchored onto the rGO substrate; and confirmed by SEM. From FTIR and XPS, those oxygen functional groups attached on rGO surface were identified and quantified. For H2S adsorption, even at the mid temperature (300 and 400 ℃) the ZnO/rGO composite showed about 3.5 times larger H2S adsorption capacity. It confirms the roles of oxygen functional groups holding metal oxide particles and it prevents the aggregation effect on the nano-sized ZnO particles. Therefore, the ZnO/rGO composite provides higher active surface area to the H2S molecules than pure ZnO.