Photosynthetic carbon fixation regulates air-sea CO2 fluxes in the waters of coral reefs. However, little has been documented on the effects of solar UV radiation (UVR, 280-400 nm) upon photosynthetic behaviors of phytoplankton dwelling in these ecosystems. In order to evaluate the aforesaid, surface dwelling tropical coral reef phytoplankton assemblages collected from the South China Sea were exposed to solar radiation (i.e., photosynthetically active radiation [PAR] + UV radiation A [UVA] + UV radiation B [UVB], 280-700 nm; PAR + UVA, 320-700 nm; and PAR, 400-700 nm) under static or simulated-mixing conditions. Under the static condition, UVA and UVB significantly reduced the carbon fixation with the maximum of 22.4 and 15.3%, respectively; while lower UVR-related photosynthetic inhibition was observed in case of phytoplankton samples being subjected to mixing. At a moderate level of mixing (i.e., circulation time 80 min), the UVA and UVB caused inhibition were lowered by 52.1 and 79.6%, respectively. Based on this it could be stated that vertical mixing induced by winds and/or tides in the natural environments could reduce the inhibitory effect of solar UVR on phytoplankton productivity in the coral reefs water.
Global warming, ocean acidification and increased UVB irradiance (280-315 nm) are known to influence marine organisms (Zepp et al. 2011). Corals, being no exception, are also subjected to these adversities (Banaszak and Lesser 2009). Coral reefs around the world have been bleached and endangered, possibly due to climate changes (Cantin et al. 2010, van Woesik and Jordan-Garza 2011, Hughes et al. 2012). Apart from various physico-chemical parameters of the environments, survival of corals also depends on several biological parameters that play vital roles in directly or indirectly affecting these reef builders. For e.g., phytoplankton in these ecosystems would be important for sustenance of coral reefs as the changes in phytoplankton photosynthetic processes in coral reef waters are known to influence stability of these ecosystems (Lesser 2004, Furnas et al. 2005). Growth of corals often makes their habitats to be oligotrophic and increase sunlight penetration (Dunne and Brown 1996, Kuwahara et al. 2010). Phytoplankton in coral reef water would thus be exposed to higher solar radiation.
Solar ultraviolet radiation (UVR, 280-400 nm) harms corals and primary producers in these waters (Banaszak and Lesser 2009). Phytoplankton species (especially those in the upper mixing layer), while utilizing light energy for photosynthesis, are also exposed to UVR that is known to reduce their growth, photosynthesis and calcification, damage DNA or D1 protein and pigments (Helbling et al. 1992, Buma et al. 2003, Bouchard et al. 2005, Roy et al. 2006, Gao et al. 2009, Li et al. 2011). Moderate levels of UV radiation A (UVA, 315-400 nm) also have positive effects, such as photo-repair of UV radiation B (UVB)-damaged DNA (Buma et al. 2003) and stimulate photosynthetic CO2-fixation at low photosynthetically active radiation (PAR) (Mengelt and Prezelin 2005, Li et al. 2011) or at PAR absence conditions (Gao et al. 2007, Li and Gao 2013). Mixing that moves phytoplankton up and down the water column causes fluctuation of the levels of solar radiation, enhancing non-photochemical quenching (Milligan et al. 2012) and lowering synthesis of UV-screening compounds (e.g., mycosporine-like amino acids [MAAs]) (Hernando et al. 2006), and also mitigating the negative effects of UVR (Neale et al. 1998). Such impacts of UVR fluctuation due to vertical mixing on phytoplankton photosynthesis have been extensively elaborated (e.g., Neale et al. 1998, Barbieri et al. 2002, Helbling et al. 2003, Milligan et al. 2012).
Coral reefs in the South China Sea are endangered as well, with the coverage decreased by 80% during the past 30 years (Hughes et al. 2012), likely being contributed by climate changes and environment degradations. Following this insight, extensive studies were conducted on these coral reefs (e.g., Li et al. 2008, Dong et al. 2009, Huang et al. 2011, Hughes et al. 2012); however, rather less attention was paid to phytoplankton in these coral reef waters (Zhang et al. 2009, Shen et al. 2010, Li and Gao 2013). As a habitat sharing counterpart to corals, phytoplankton may indirectly affect the survival of corals (discussed earlier); in view of this point, it is essential to evaluate the physiological effects of environments on these organisms. Here, we focus on examining the impacts of solar UVR on photosynthetic carbon fixation of phytoplankton assemblages from a coral reef water of the South China Sea and its relationship with mixing.
A coral reef region of Xisha Islands in the South China Sea (Fig. 1), where about 127 species of corals (Li et al. 2008) and more than 150 species of coral reef fishes (Sun
et al. 2005) were recorded, was chosen as the experimental area. Surface seawater samples (20 cm depth) were collected at a site 300 m off Yongxing Island (16°51′ N, 112°20′ E) (the largest island amongst the Xisha Islands [~1.8 km2]), using a 10 L acid-cleaned (1 N HCl) polycarbonate container from 23 March to 1 April (Julian day 81 to 90) of 2007. Sample collections were carried out four times each day. The water samples collected in the morning (8:30 AM) were used for photosynthetic measurement and treated for chlorophyll
Sea surface temperature (SST), salinity (SSS) as well as wind speeds were obtained from Xisha Oceanic Monitoring Station located 100 m off the sampling site. Surface pH value was measured with an Okaton pH meter (Vernon Hills, IL, USA).
Incident solar radiation was continuously monitored using a diving broad-band radiometer (ELDONET; Real Time Computers Inc., Mohrendorf, Germany). This device efficiently measures the terrestrial or underwater solar irradiance of three ranges of UVB (280-315 nm), UVA (315-400 nm), and PAR (400-700 nm) (Hader et al. 1999). It is also equipped with a temperature and depth sensor to obtain the underwater profiles.
Photosynthetic carbon fixation by phytoplankton assemblages was measured by dispensing pre-filtered seawater (180 μm-pore mesh) into 50 mL quartz or glass tubes, inoculating NaH14CO3 solution (see below) and exposing to static or simulated-mixing conditions as described below with three radiation treatments (triplicate for each): a) PAR + UVA + UVB (PAB, 280-700 nm), uncovered quartz tubes; b) PAR + UVA (PA, 325-700 nm), uncovered glass tubes (50% transmittance at 325 nm); and c) PAR alone (P, 400-700 nm), quartz tubes wrapped with Ultraphan UV Opak Digefra film (50% transmittance at 395 nm) (UV Opak; Digefra, Munich, Germany). The transmission spectra of the tubes or cut-off foils and measurable errors have been reported previously (Li et al. 2011).
A time-series (days 81 to 90) of the impact of UVR exposure on photosynthesis was measured under static condition, through directly incubating phytoplankton samples in the three treatments in a water tank of 1.5 m × 1.0 m × 0.2 m (described below).
Effect of vertical mixing caused fluctuation on UVR exposure was determined by carrying out two sets of experiments: one set (days 89, 90) was comprised of 7 mixing depths, i.e., 0 (static condition), 3.2, 6.7, 10.2, 14, 16, and 21 m (close to bottom of euphotic zone) (Fig. 2). The fluctuated irradiance that the samples received was simulated by continuously manipulating the different covering layers of neutral screens and simultaneously altering the solar radiation levels. For e.g., 3.2 m mixing depth, one layer of screen was covered and removed with a 20 min interval, fluctuating the cell-received irradiance from 100 to 55% and then backwards up to 100%; and for e.g., 21 m mixing depth, 6 layers of screens were covered one by one with a 20 min interval, decreasing the irradiance stepwise from 100, 55, 25, 15, 6, 3 to 1.5% and then removed the screens one by one to backwards up to 100% of surface sunlight. The other set (days 86, 87), a simulated 14 mmixed- depth was designed to determine the effects of UVR at varying mixing frequencies on the photosynthesis, i.e., 4 layers of neutral screens were covered then removed one by one, fluctuating the cell received irradiance stepwise from 100, 55, 25 to 15% of surface sunlight then backwards up to 100%; here, the intervals of changing screens were set at 5, 10, 20, and 30 min for each different incubation, making the circulation time of 40, 80, 160, and 240 min, respectively. We are aware that using stacks of neutral screens to block sunlight does not mimic the differential attenuation of UVR and PAR in water column,
but it indeed provides very useful information to accomplish the objective of this study.
>
Photosynthetic carbon fixation
Each of the aforesaid 50 mL sample was inoculated with 100 μL of 5 μCi (0.185 MBq) NaH14CO3 solution (ICN Radiochemicals, Irvine, CA, USA). All the three sets of the aforesaid tubes containing phytoplankton samples were incubated for 6 h (9:30-15:30) under solar radiation in a tank continuously flushed with surface seawater at 25- 30℃ in order to mimic the natural environmental conditions. These samples were then filtered onto a Whatman GF/F glass fiber filter (25 mm in diameter) which was placed into a 20 mL scintillation vial; followed by this, they were exposed to HCl fumes overnight and dried to expel the non-fixed 14C. A fixed volume (3 mL) of scintillation cocktail (UltimaGold; Perkin Elmer, Waltham, MA, USA) was added to each vial and the incorporated 14C was measured with a liquid scintillation counter (LS 6500; Beckman Coulter, Fullerton, CA, USA). The carbon fixation rate of phytoplankton assemblages was calculated according to Holm-Hansen and Helbling (1995).
Chl
For taxonomic analyses, the seawater samples (50 mL each) were fixed with buffered formalin (final concentration of 0.4%) and allowed to settle for 24 h in a cylinder of Utermohl Chamber (Hydro-Bios, Kiel, Germany). Phytoplankton species were identified and enumerated with the aid of an inverted microscope (IX51; Olympus, Tokyo, Japan).
Inhibition of photosynthetic carbon fixation or productivity due to UVR exposure was calculated based on the following formulae (Helbling et al. 1992):
Inh-UVA (%) = (PP - PPA)/PPAB × 100%
Inh-UVB (%) = (PPA - PPAB)/PPAB × 100%,
where Inh-UVA and Inh-UVB are the inhibition caused by UVA or UVB; PP, PPA, and PPAB are the photosynthetic production or carbon fixation rate under PAR-alone, PAR + UVA, and PAR + UVA + UVB, respectively.
Paired t-test was used to determine the significant differences among PAB, PA, and P treatments; one way ANOVA was used to establish the significant differences of UVR effects between under varying light and static conditions (control). The correlation analyses between the variables were established using a Kendall’s τ test with 95% confidence limit.
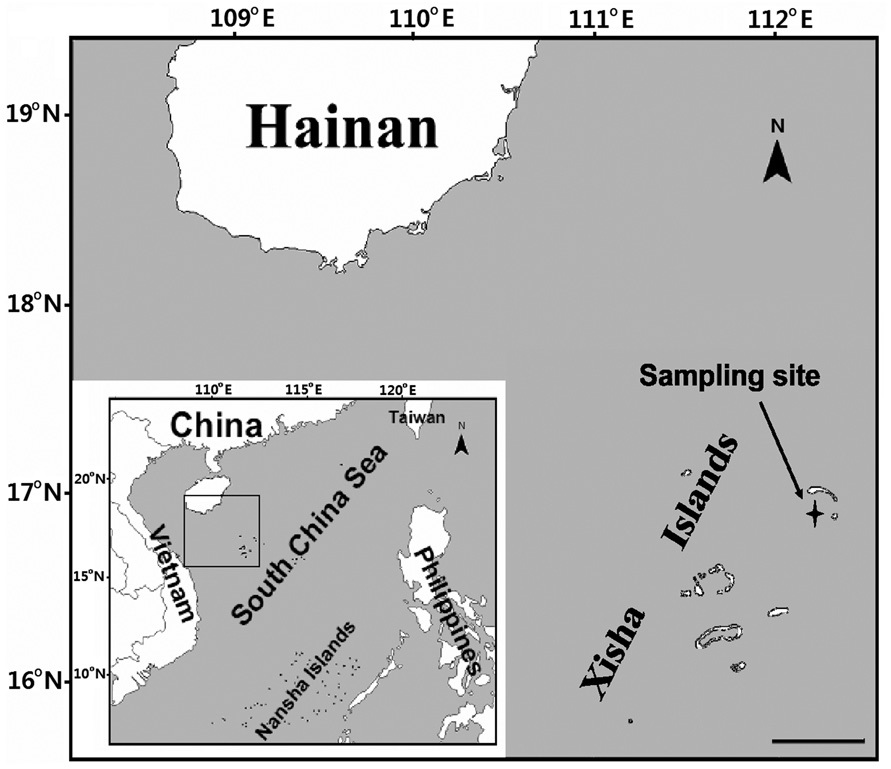
Typical profiles of sunlight (Fig. 2) obtained on Julian day 82 showed the changes of solar irradiance with increasing water depth, wherein the lower limit of euphotic zone reached 22 m (K, 0.2 m-1); whereas 1% of surface
UVA and UVB penetrated to 12 m (K, 0.40 m-1) and 8.4 m (K, 0.55 m-1), respectively.
Total ozone column concentration over Yongxing Island varied from 251 to 255 Dobson Units from Julian day 80 to 92 (http://jwocky.gsfc.nasa.gov/). Daily PAR doses of 4.9-9.3 MJ m-2, UVA of 0.83-1.46 MJ m-2 and UVB of 33.6-61.7 KJ m-2 were recorded. SST and SSS ranges of 25.3-29.7℃ and 33.8-34.2 were recorded, and the ranges of pH and day-averaged wind speed were 8.18-8.36 and 1.95-5.77 m s-1, respectively.
Total Chl
cells accounted for maximal proportion of Chl
Photosynthetic ability of phytoplankton assemblages recorded under maximal solar radiation ranged from 1.0 to 4.89 μg C L-1 h-1, on days 82 and 88, respectively (Fig. 3B); whereas photosynthetic rate varied from 4.06 to 7.74 μg C (μg Chl
Exposing phytoplankton assemblages to changing irradiance (i.e., alter sunlight level stepwise from 100 to 1.5%, then back up to 100%) under mixing regimes with mixing depths increasing from 0 to 16 m, caused an increase in photosynthetic rate (PAB treatment), i.e., an increase of 5.07 to 6.87 μg C (μg Chl
At a fixed-mixing depth of 14 m, when the mixing frequencies decreased (by prolonging the intervals of covering or removing screening filters from 5 to 30 min) and cells assembly received the same doses of sunlight, the photosynthetic rate (PAB) sharply decreased from 6.98 to 5.21 μg C (μg Chl
Coral reefs are generally distributed in shallow tropical waters (Stone 2006), and are often subjected to tides and / or wind induced vertical mixing. Here, we demonstrated that moderate mixing lowered the UVR caused inhibition on photosynthesis of phytoplankton assemblages in the coral reef waters of the South China Sea; moreover, photoinhibition was not only caused by UVR but also PAR, even when the mixing reached as deep as 16 m in this area.
More resistance of phytoplankton to UV radiation, indicated by lower UVR caused photoinhibition (Fig. 3D), was recorded in this coral reef water, as compared to that of the cell assemblies from coastal water for the same time period (Wu et al. 2010). It would be attributed by the long term high solar radiation and light penetration (Fig. 2) since the higher light exposure could make the cells induce more efficient defensive or repair mechanisms involved in the protection and repair processes e.g., synthesizing and accumulating the MAAs and antioxidant enzymes (Hernando et al. 2006). Higher temperature (~28℃) in this water could be another cause for depressing the inhibitory effects of UVR, likely attributed by the enhancing activities of enzymes involved in the processes of damage-repair or photosynthesis (Buma et
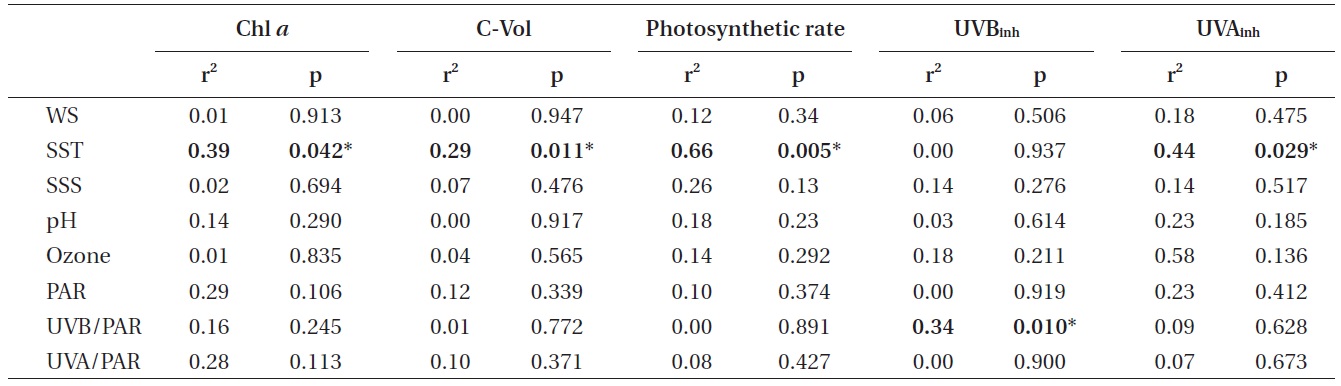
r2 and p-values of various parameters studied plotted against the environmental parameters
al. 2003). This fact can also explain the reason why higher photosynthetic productivity was observed at higher temperatures, i.e., the carbon fixation rate was positively correlated to SST (Table 1).
In natural marine environments, it is obvious to understand that mixing would cause a change in optical property in the water column, thereby influencing quantity and quality of solar irradiance therein. Coral reef waters are often well mixed due to the tides and / or winds (Gardner et al. 2011). Changes in UVR levels like those produced by mixing can affect the performance and fitness of aquatic organisms (Hebling et al. 2003, Hernando et al. 2006). Depth and intensity (rate) of mixing had a profound effect on the UVR induced damage to photosynthesis of phytoplankton, as shown here (Fig. 4) or in other studies (Barbieri et al. 2002, Hebling et al. 2003). Guan and Gao (2008) have formerly reported that the intensity and duration of UVR exposure are critical to balance the UVR damage and repair processes. As the mixing depth increased, phytoplankton were exposed more periodically to lower levels of solar radiation; the UVR damages could thus be counteracted by repair processes, resulting in the higher photosynthetic rate and lower UVR induced inhibition (Fig. 4A & C). Simultaneously, the increasing photosynthetic ability with mixing depth also indicated that not only solar UVR but PAR photoinhibited the carbon fixation of phytoplankton assembly, even when the mixing deepened to 16 m (Fig. 4A). When the intensity of mixing was kept moderate (80 min per circulation) for a certain time interval (6 h) and the frequency of light exposure varied, i.e., high / low, a decrease in UVR inhibition was noted, as compared to the slow-mixed, fast-mixed or static samples (Fig. 4D). This indicates that a certain time period is necessary to balance the UVR caused damage and to initiate the repair processes (Neale et al. 1998, Guan and Gao 2008).
Based on the study conducted here (Fig. 4), it could be stated that apart from the intensity or dose of solar irradiation and its dynamics (Cullen and Lesser 1991, Gardner et al. 2011), there are several other environmental parameters such as mixing which play a pivotal role in and account for the extent of UVR induced inhibition on photosynthesis of phytoplankton assemblages from the coral reefs. The mixing that usually prevails the coral reef water would surely help in combating UVR harms on phytoplankton therein; this would in turn help establish a conducive environment for corals and would help them to flourish.
![(A) Total phytoplankton biomass, chlorophyll a (Chl a, μg L-1) and percentage (%) of Chl a in pico- and nanophytoplankton fraction (<5 μm). (B) Carbon fixation capacity (μg C L-1 h-1) and (C) photosynthetic rate (μg C [μg Chl a]-1 h-1) of phytoplankton assemblages exposed to photosynthetically active radiation (PAR) + UV radiation A (UVA) + UV radiation B (UVB) (PAB, 280-700 nm), PAR + UVA (PA, 325-700 nm), and PAR (P, 400-700 nm). (D) UVA or UVB caused inhibition (%). Vertical bars represent standard deviations (n = 3).](http://oak.go.kr/repository/journal/12569/JORHBK_2013_v28n3_281_f003.jpg)
![Photosynthetic rate (μg C [μg Chl a]-1 h-1) of phytoplankton assemblages (A) at various mixing depths or (B) at a fixed depth with different mixing frequencies under photosynthetically active radiation (PAR) + UV radiation A (UVA) + UV radiation B (UVB) (PAB, 280-700 nm), PAR+UVA (PA, 325-700 nm), and PAR-alone (P, 400-700 nm). The inhibition (%) due to UVA or UVB exposure at various mixing depths (C) and at a fixed depth with varying mixing frequencies (D). Vertical bars represent standard deviations (n = 3 or 6).](http://oak.go.kr/repository/journal/12569/JORHBK_2013_v28n3_281_f004.jpg)