The White Sea is a cold temperate extension of the polar Barents Sea and Arctic Ocean. There is a convoluted shoreline of over 3,000 km with numerous islands and inlets, and an opening to the Barents Sea to the northeast 45-65 km wide and 150 km long km. Numerous rivers provide extensive freshwater input, and salinities can be very low even at some distance from river mouths. The relatively shallow slopes provide for extensive intertidal and subtidal shores where benthic algae can grow (Zinova 1929, 1950, Vozzhinskaya 1986, Mikhaylova et al. 2010). Thus there is a wide diversity of habitats suitable for macroalgae, including wave-exposed to protected rocky shores, and subtidal sites supporting extensive kelp beds (e.g., Myagkov 1975, Mikhaylova 1996). There is also a range of estuarine habitats from near freshwater sites with ca. 1.5 m tidal amplitudes, to salt marshes covering many hectares. While the White Sea lies on the Arctic Circle, summer surface temperatures can reach 18℃, and ice conditions are limited to 4-5 months in winter and spring (Tolstikov and Petrov 2006). A significant constraint on algal growth at high latitudes is the relative absence of light from late fall through winter months (Wiencke et al. 2007, Wulff et al. 2009). The White Sea is surrounded on all sides by boreal forest. Thus, the White Sea is an example of a temperate water body that has been relatively isolated during the Holocene, and which is not part of a continuous arctic to tropical climatic gradient.
The limited algal diversity of northern Russia has long
been recognized (Kjellman 1883, Luning 1990, Vinogradova 1995). This is based on the paucity of suitable substrata and the inflow of freshwater along the extensive northern coast. The White Sea is an exception to this generalization, and a long history of floristic studies show lush populations of macroalgae. The principal algal monographs including Zinova (1929, 1950, 1953, 1955), Vozzhinskaya (1986), and Mikhaylova et al. (2010) are supplemented with more comprehensive taxonomic and ecological studies on individual taxa (e.g., Perestenko 1965, Schoschina 1996, Mikhaylova 2000b). While there are numerous floristic notes from the White Sea as a whole (e.g., Kalugina 1957, 1959a, Petrov 1967, Vozzhinskaya 1975, Shoshina 1979, Vinogradova and Shtrik 2005), the Keret Archipelago in Kandalaksha Bay has received little attention from specialists with the objective of producing a more comprehensive floristic treatment. Preliminary works by Balashova et al. (2005) and Mikhaylova (2010) provide floristic background material for the Keret Archipelago and the White Sea as a whole. This is supplemented by ecological (e.g., Myagkov 1975, Tarakhovskaya and Garbary 2009, Maximova and Sazhin 2010), and physiological studies for the region (e.g., Tarakhovskaya and Maslov 2005, Berger 2009).
Most of the primary literature on seaweeds of the White Sea has been published in Russian and is not widely disseminated outside of Russia. Here we provide a floristic list of the seaweeds of the Keret Archipelago based primarily on collections in August 2008. We put these records into a context of the relevant Russian literature on the distribution and ecology of these species in the White Sea as a whole, and provide brief comments on species of special note.
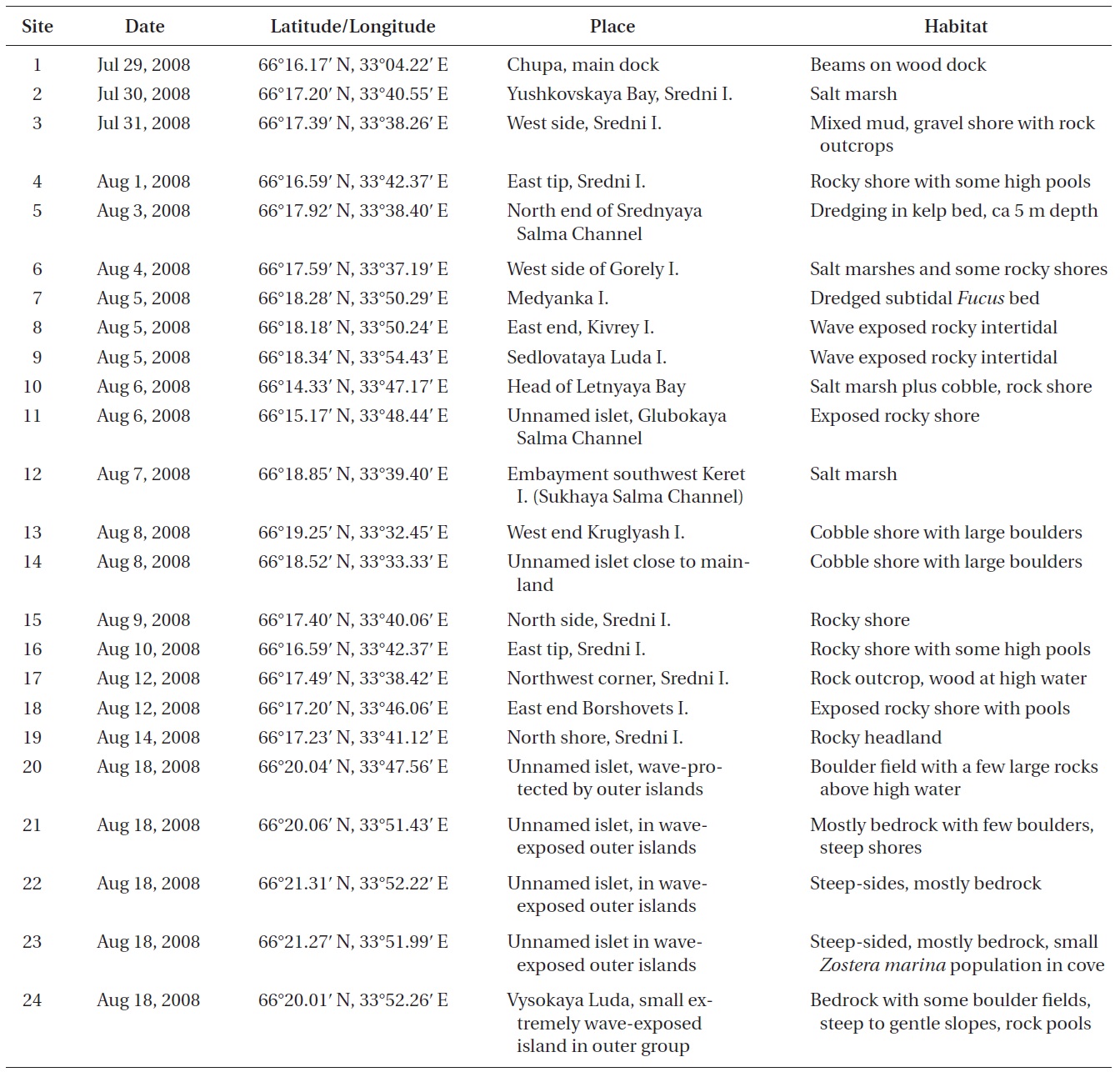
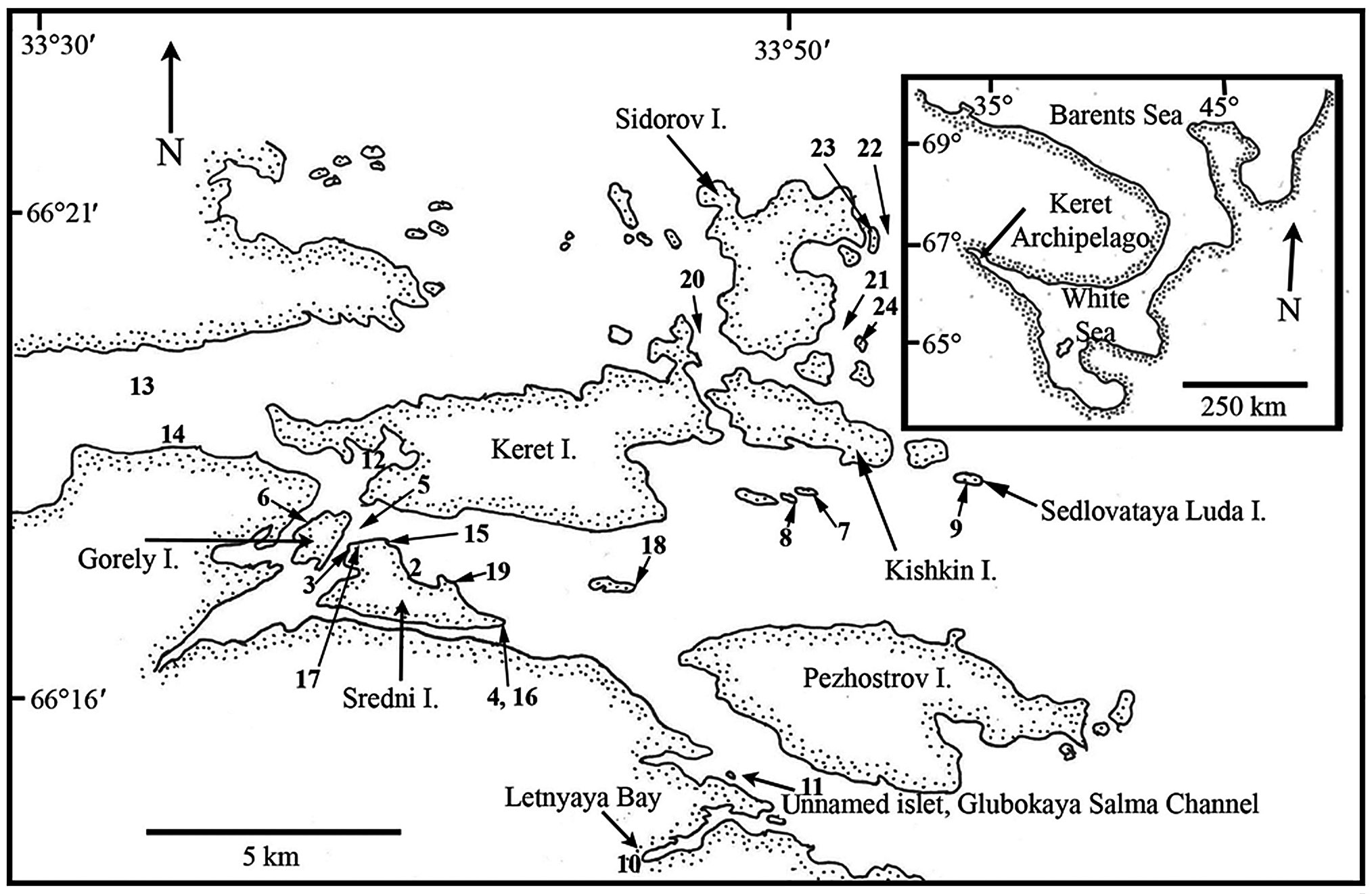
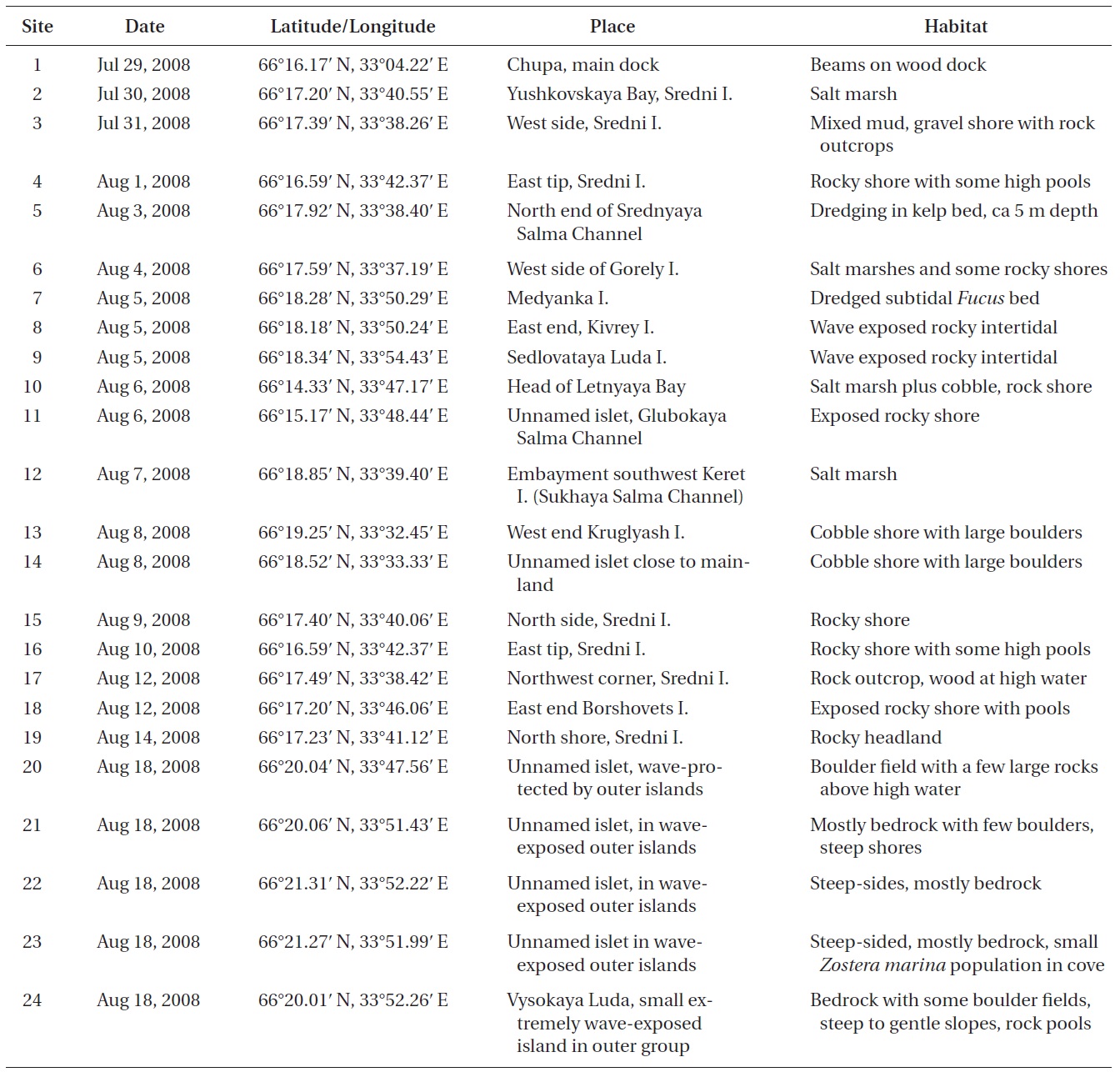
Sites in the Keret Archipelago and adjacent continental area of Kandalaksha Bay in the White Sea were visited during late July and August 2008 (Table 1, Fig. 1). Collection sites varied from very low salinity brackish areas (<5 psu) to exposed open coasts with salinity >25 psu. The temperature regime of surface waters in near-shore coastal areas varies annually from -1.8 to 18.7℃ (Tolstikov and Petrov 2006). A variety of habitats were sampled by routine inspection of shorelines and dredging of subtidal kelp and Fucus beds (Table 1). In many cases, collections were not made of common species, although their presence was generally recorded in field notes. Voucher specimens are deposited in the Komarov Botanical Institute (K) with duplicates in the herbarium of St. Francis Xavier University (STFX). Green algae were primarily identified using Brodie et al. (2007) and nomenclature follows AlgaeBase (Guiry and Guiry 2012). The entire list of Keret Archipelago algae is given in Table 2 and remarks on species of special interest are given in the Results.
Blidingia minima (Nageli ex Kutzing) Kylin. This species was included in the list for the Keret Archipelago (Balashova et al. 2005), although the limited distribution of that publication meant that this record for the White Sea was not generally recognized. While culture studies
are often needed to distinguish some taxa of Blidingia (e.g., Garbary and Tam 1989), B. minima is morphologically distinctive. The current records (Table 2) are a confirmation that B. minima occurs in the White Sea. We found B. minima at four sites, in all cases in the high intertidal zone in wave-exposed locations. Despite searches in more typical brackish water habitats, further collections were not found, nor were other species of Blidingia. B. minima is common in the Keret Archipelago, but its
habitat seems to be restricted relative to the North Atlantic Ocean (Brodie et al. 2007).
Balashova et al. (2005) also included B. chadefaudii (J. Feldmann) Bliding in their list based on the occurrence of slightly thickened walls, however, we were unable to verify this by further collecting. Given the problematic nature of B. chadefaudii (see Brodie et al. 2007, for discussion), we omit it from the list pending further investigation.
Capsosiphon fulvescens (C. Agardh) Setchell et Gardner. Three collections of this often-overlooked alga were made on Sredni and Gorely Islands. All three populations had the golden-yellow to brownish walls reported previously (Garbary et al. 1982). It was the coloured walls that allowed thalli of one collection to be distinguished from the much larger populations of Ulva intestinalis with which it was generally associated. It was found both on wood and rock substrata in the high intertidal zone. This species has been reported twice previously from the White Sea (Zinova 1961, Mikhaylova and Shtrik 2007).
Chaetomorpha melagonium (Weber et Mohr) Kutzing. Our collections suggest that C. melagonium is the only large-celled member of the genus in the Keret Archipelago. Balashova et al. (2005) include C. linum (Muller) Kutzing and C. aerea (Dillwyn) Kutzing, and Myagkov (1975) includes C. aerea from Chupa Bay. These are now regarded as synonymous (Brodie et al. 2007). While an initial identification of our material suggested C. aerea, based on cylindrical cell shape and large size of the attachment cell, we concluded that the material was C. melagonium. Some filaments were also observed unattached, entangled with the holdfast of drift Laminaria digitata.
Cladophora glomerata (Linnaeus) Kutzing, and Cladophora fracta (O. F. Muller ex Vahl) Kutzing. Several species of primarily freshwater algae were included in Balashova et al. (2005) that occur in brackish environments, and two are retained in the current list: Cladophora fracta and Cladophora glomerata. Local phycologists refer to this material as C. glomerata, whereas Myagkov (1975) refers to C. fracta in Chupa Bay. These species are recognized as being able to penetrate into brackish systems (e.g., Brodie et al. 2007), and free-floating skeins of a Cladophora are abundant in the intertidal and shallow subtidal zones, particularly in areas influenced by the Keret River. Thus some of the islands in the Keret Archipelago have an interesting mix of freshwater Cladophora and the clearly marine Fucus vesiculosus, Ascophyllum nodosum, and Hildenbrandia rubra. Using the key in Brodie et al. (2007) some of these Cladophora skeins keyed out to C. liniformis Kutzing; however, this is within the form range of C. glomerata.
Eugomontia sacculata Kornmann. This was the only endozoic form that could be identified from green tinted bivalve shells. Collections were from pools in salt marshes. Our identification of this species represents a new record for the White Sea. Based on the number of green shells we observed, the species is expected to be common in the White Sea.
Prasiola calophylla (Carmichael ex Greville) Kutzing.
Prasiola crispa (Lightfoot) Kutzing.
Prasiola furfuracea (Mertens ex Hornmann) Kutzing.
Colonies of all three Prasiola spp. were typically found as expanses of single species on high intertidal boulders that projected well above the surrounding intertidal zone, either in salt marshes or on boulder fields with extensive fucoid populations. When present, the populations formed extensive green carpets on the rock surface or in rock crevices. While the elevation may have been above spring tides, the sites would all have been within the splash zone.
Rhizoclonium riparium (Roth) Harvey. This species was very common in the salt marshes where it formed mats in the high intertidal zone. It was also found in the high intertidal zone on rocky shores where there is sometimes a fringe of marsh vegetation. On more exposed rocky shores small tufts may occur in crevices in the high intertidal zone. R. riparium was not listed in Balashova et al. (2005); however, Myagkov (1975) reported the species in Chupa Bay. This species is widely reported from the White Sea and has been discussed under various names including Rhizoclonium hieroglyphicum (C. Agardh) Kutzing and R. rigidum [sic] (e.g., Vozzhinskaya 1986).
Rosenvingiella radicans (Kutzing) Rindi, McIvor and Guiry. The single collection of this species from Chupa dock is a new distribution record for the White Sea.
Ulothrix implexa (Kutzing) Kutzing. Several collections were found on both a rocky shore and in a salt marsh. The material agrees with the description of Brodie et al. (2007). This is a new record for the White Sea.
Ascophyllum nodosum (Linnaeus) Le Jolis. This species is abundant and common on most shores ranging from salt marshes to rocky shores. On the latter, scattered thalli occur among the Fucus vesiculosus, and it tends to form a band in the low intertidal and shallow subtidal zones. This species is well known in White Sea (e.g., Kalugina 1958, Vozzhinskaya 1986, Maximova and Sazhin 2010).
Ascophyllum nodosum f. scorpioides Hauck. The extensive salt marshes of the Keret Archipelago yield the unattached form of Ascophyllum, A. nodosum f. scorpioides. This form has been referred to several times from the White Sea (Zinova 1950, Kalugina 1958, Vozzhinskaya 1986), but its occurrence in the Keret Archipelago merits comment. This unattached form was abundant in several marshes where it occurred virtually throughout the intertidal zone. These thalli varied from 5-50 cm in diameter. In the lower and mid-intertidal zones more open branched thalli with remnants of a central axis were intermixed with abundant F. vesiculosus, the latter growing attached to rocks. Higher in the marshes f. scorpioides often occurred as densely branched thalli with thin axes that curved upwards. These thalli did not have the developing receptacles that were evident on the attached populations.
Chordaria flagelliformis (Muller) C. Agardh. Chordaria flagelliformis is common in the Keret Archipelago (Balashova et al. 2005), although it is more prominent in the outer islands where it forms a distinct band on ice-scoured shores. This species is widely reported from the White Sea (e.g., Mikhaylova 2000a).
Elachista fucicola (Velley) Areschoug. This is a common epiphyte of Fucus vesiculosus and A. nodosum. Like Nova Scotia, it seems to be the preferred habitat for the marine chironomid Halocladius variabilis (Garbary et al. 2009, Tarakhovskaya and Garbary 2009).
Fucus distichus Linnaeus. The taxonomy of F. distichus is complex and depending upon the treatment may include F. evanescens C. Agardh and F. edentatus Bachelot de la Pylaie (e.g., Kucera and Saunders 2008). The taxonomic concept used here is that F. distichus refers to those forms that inhabit high intertidal rock pools. Populations of such thalli were observed at least three times on exposed rocky shores. This species is widely reported from the White Sea and Balashova et al. (2005) uses F. filiformis Gmelin.
Fucus vesiculosus Linnaeus. Fucus vesiculosus is the most characteristic species of seashores in the Keret Archipelago. It occurs in all habitats from highly brackish systems in tidal river channels, to salt marshes and rocky shores where it is found from the low to high intertidal zones. As a consequence of this habitat range, the plants are highly variable in size, colour, and vesiculation. A number of these forms have been ascribed different names and Balashova et al. (2005) uses F. inflatus Vahl, a taxon typically synonymized with F. vesiculosus.
A subtidal form of F. vesiculosus occurred off Medyanka Island among the outer islands of the archipelago. Thalli were often >1 m long. While the paired bladders of F. vesiculosus were conspicuous, the population was not part of a continuous Fucus band from the intertidal zone, but formed a separate population below a band of Ascophyllum nodosum. Since the size of these plants and their subtidal habitat are unusual for F. vesiculosus, this population should be examined using molecular approaches to determine the extent of genetic separation.
Pelvetia canaliculata (Linnaeus) Decaisne et Thuret. This highly distinctive species was found at three sites, and it is likely present on exposed shores of all the outer islands. The populations had the characteristic morphology and occurred in the same high intertidal zone on rocky shores as in the eastern North Atlantic; however, the thalli were mostly 5 cm or less tall, and the receptacles were <5 mm long.
Saccharina latissima (Linnaeus) Lane, Mayes, Druehl & Saunders. This is the common kelp of the subtidal zone in the Keret Archipelago. In the inner islands it tends to form wide blades that are very brittle, whereas in more exposed and higher salinity conditions it forms more typical narrow blades with ruffling.
Acrochaetium virgatulum (Harvey) Batters. Only a few thalli were identified as A. virgatulum sensu Dixon and Irvine (1977), but the axial stellate chloroplasts with single pyrenoids, the discoid base and straight branches are characteristic features. This species can be confused with A. secundatum (Lyngbye) Nageli, and these species have been proposed for synonymy; however, none of our material had the secund branching typical of A. secundatum. Careful search of for the latter did not reveal any thalli, although it is reported from elsewhere in the White Sea (e.g., Zinova 1950).
Ahnfeltia plicata (Hudson) Fries. This is the most abundant red alga of the low intertidal zone where it occurs in pools and as an understory, and in the subtidal zone where it forms large beds. Where one would expect to see Chondrus crispus Stackhouse in the North Atlantic, A. plicata is typically present. It occurred at most sites as drift. Ahnfeltia plicata has been reported widely from the White Sea including the Keret Archipelago (e.g., Zvereva 1938, Mikhaylova 2000a).
Ceramium virgatum Roth. This is a common alga of the subtidal zone and it has been widely reported from the White Sea. The record of C. deslongchampsii Chauvin ex Duby from Chupa Bay (Myagkov 1975) and reports of several other Ceramium species from the White Sea [e.g., C. arborescens J. Agardh, C. areschougii Kylin, C. circinatum (Kutzing) J. Agardh and C. secundum Lyngbye] may be part of the morphological variation in C. virgatum that occurs as a consequence of growth in different daylengths (Garbary et al. 1978).
Coccotylus truncatus (Pallas) Wynne et Heine. Coccotylus truncatus is common in the Keret Archipelago and it is widely reported from the White Sea. Balashova et al. (2005) included it under the older name Phyllophora brodiaei (Turner) Endlicher. This species was revised by Wynne and Heine (1992). C. truncatus resembles C. crispus Stackhouse, and has a similar ecological range, occurring in rock pools, as an understory plant in the low intertidal zone and as more continuous stands in the subtidal zone.
Colaconema daviesii (Dillwyn) Stegenga. This species was seen twice as an epiphyte on other red algae. The parietal chloroplast with a single chloroplast in each cell, the small clusters of monosporangia and the absence of attenuate hairs distinguish this entity from most others in the complex. This species has been reported from the White Sea (Mikhaylova and Shtrik 2007), although the current collections may be more definitive.
Grania efflorescens (J. Agardh) Kylin. Grania efflorescens was the most common epiphytic member of the ‘acrochaetioid’ complex in the Archipelago, and was included in the list of Balashova et al. (2005). The thalli were poorly branched, and the relatively long, narrow cells with parietal chloroplasts are devoid of pyrenoids. The numerous records from the White Sea suggest that it is widely distributed and common.
Harveyella mirabilis (Reinsch) Schmitz et Reinke. This parasite was common on Rhodomela confervoides from subtidal collections where it was noted in at least two collections. Kalugina (1959b) provided a description from the White Sea, and the species is expected wherever the host is common.
Palmaria palmata (Linnaeus) Weber & Mohr. Palmaria palmata was very common. On exposed shores scattered thalli were attached to rock, and it was a common element in the drift throughout the Keret Archipelago. Mikhaylova and Shtrik (2007) discussed the community ecology in the White Sea.
Polysiphonia spp. Three species of Polysiphonia were commonly encountered: two uncorticated species with four and six pericentral cells [P. stricta (Dillwyn) Greville and P. arctica J. Agardh], and a corticated species with many pericentral cells [P. fucoides (Hudson) Greville]. These have all been reported from the Keret Archipelago although names used by Myagkov (1975) and Balashova et al. (2005) differ, and one species, Vertebrata lanosa [as Polysiphonia fastigiata (Roth) Greville] is likely based on a misidentification of P. fucoides. Vertebrata lanosa is the obligate epiphytic red alga generally restricted to A. nodosum in the North Atlantic Ocean (e.g., Garbary et al. 1991). Despite a careful search of A. nodosum thalli in all habitats from salt marshes to exposed rocky shores, this species was not found. Since this species is unknown elsewhere in the White Sea, and is conspicuous when present, it seems an unlikely member of the flora.
Rhodochorton purpureum (Lightfoot) Rosenvinge. Rhodochorton purpureum is previously known from the Keret Archipelago and from the White Sea. We observed an extensive population on Kivrey Island where it formed a dense mat as an understory to Fucus vesiculosus and Ascophyllum nodosum. It also occurred as a sporadic epiphyte on various subtidal algae.
Rubrointrusa membranacea (Magnus) Clayden & Saunders. Rubrointrusa membranacea is a common endozoic species in the hydroid Dynamena pumilla (Linnaeus), and several collections were made. While the material was not reproductive, the habit, chloroplast morphology and often-irregular cell shape are diagnostic (Clayden and Saunders 2010). This is the first record for the Keret Archipelago but the species is well known from the White and Barents Seas (e.g., Kalugina 1962).
Wildemania (Porphyra) amplissima (Kjellman) Foslie. Balashova et al. (2005) suggested the presence of Porphyra in the Keret Archipelago and at least six specific names have been used for White Sea. We observed Porphyra only on the most exposed islands of the Archipelago where it was abundant on wave-swept shores above a zone of Chordaria flagelliformis. Fronds of P. amplissima were mostly around 10 cm long and 2-3 cm wide. The material was not fertile.
Vaucheria sp. The genus Vaucheria (Tribophyceae) has over 50 species world-wide, occurring mostly in freshwater habitats. A number of species are characteristic of salt marshes and brackish water conditions in general. For example, Pankow (1971) described ten species of Vaucheria from the Baltic Sea, and many of these species are widespread in the Atlantic and Pacific Oceans (e.g., Garbary and Fitch 1984). None of these species have been found in the White Sea. Here we report a Vaucheria species from a mainland salt marsh adjacent to the Keret Archipelago. The deep green mats occurred high in the salt marsh but below the elevation of extensive colonies of Rivularia atra Roth ex Bornet and Flahault at the site. The sediment at this location was quite sandy as opposed to the grey clay and silty muds that characterize most of the other marshes observed during this study.
The two Vaucheria colonies observed were about 4 cm in diameter in situ and about 1 m apart. The filaments were about 55 μm diameter.
Outside of salt marshes and seagrass beds one does not normally associate flowering plants with marine algal communities. In the Keret Archipelago flowering plants are a regular occurrence on gravel and rocky shores where there is some sediment deposition. We discuss these species briefly because they are such an unusual part of the upper and mid intertidal zones relative to temperate North Atlantic communities.
Aster tripolium Linnaeus. Aster tripolium is a regular member of the salt marsh and upper rocky shore community in the Keret Archipelago. On Sredni Island on both the brackish and fully marine shores, A. tripolium occurs as a member of the intertidal community. It occurred with Fucus vesiculosus on cobble and gravel shores, and also with Ascophyllum nodosum on more muddy shores, including salt marshes. While members of the Asteraceae may occur in salt marshes in the North Atlantic, they are not typical members of the rocky intertidal community.
Plantago maritima Linnaeus and Triglochin maritima Linnaeus. These species have very similar form in terms of leaf shape and arrangement and the form of the inflorescence. Both have succulent, needle shaped leaves and a single flowering spike; however, the leaves and inflorescences are considerable smaller in P. maritima. The Keret Archipelago has extensive stretches of rocky to gravel shores where T. maritima, and P. maritima to a lesser extent, are abundant alongside F. vesiculosus in the mid and upper intertidal zones. In low salinity areas T. maritima can extend to the lower intertidal zone. T. maritima is also a dominant plant in salt marshes where it seems to replace the Spartina alterniflora that is typical of marshes in the northwestern Atlantic.
Zostera marina Linnaeus. Zostera marina is common in the Keret Archipelago. It occurs primarily is sites influenced by freshwater where it forms a band in the shallow subtidal immediately below Ascophyllum nodosum. In some shallow bays it forms more extensive populations, and it sometimes occurs in the intertidal zone when it is exposed during spring tides. While we collected leaves of Z. marina to examine for epiphytes, they were generally free of epiphytic macroalgae except for the ubiquitous skeins of Cladophora glomerata. Kalugina (1958) and Vozzhinskaya (1986) discuss community ecology of Z. marina in the White Sea.
Zostera noltei Hornemann. Zostera noltei was common on many shores of the inner islands where mud and clay occurred in the intertidal zone. This species occurred in dense patches on soft bottoms in very shallow pools or in thin films of surface water. When exposed by the tides, the plants were flat on the substratum. The plants were no more than 5-10 cm long and were commonly reproductive. On adjacent rocks it was common to find Fucus vesiculosus. Kalugina (1958) discusses community ecology (as Z. nana) in the White Sea.
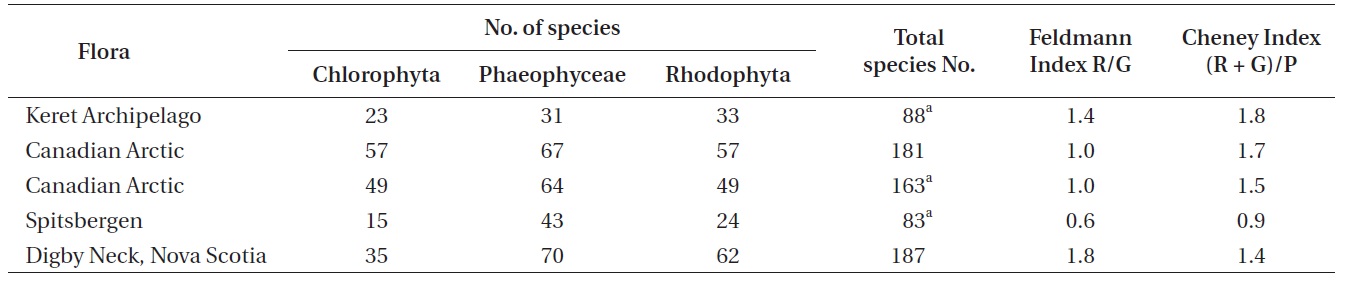
Together with the 56 species of algae listed in Balashova et al. (2005) the new list for the Keret Archipelago comprises over 80 species that can be generally recognized (Table 2). This list includes 23 species of Chlorophyta, 31 species of Phaeophyceae, 33 species of Rhodophyta and one species of Tribophyceae. Of the 88 species now included, the authors observed over 60 during this study. In addition, over 20 of the species were not included in Balashova et al. (2005), and seven species are new records or important confirmations of prior records for the White Sea. The species that have been added are basically temperate species that are widely distributed in northern Europe. The number of new records for the White Sea point to the need for further floristic study of this region, in particular, greater seasonal and depth sampling, and more extensive surveys of exposed shores.
The occurrence of the three subterrestrial, western European species of Prasiola, and the absence of the more marine P. stipitata Suhr ex Jessen in the White Sea provides a curious anomaly. Thus, how did these species disperse, when P. stipitata that has an ecological range much lower in the intertidal zone fail to do so? We hypothesize that the apparent absence of P. stipitata argues against a post-glacial dispersal around Scandinavia and through the Barents Sea into the extreme western portion of the White Sea. Furthermore, we suggest that the occurrence of the three Prasiola species raises the possibility of wind dispersal of spores. This is consistent with the widespread occurrence of these species on terrestrial walls and moist terrestrial habitats in general in western Europe (e.g., Rindi and Guiry 2004, Rindi 2007).
There are no endemic species currently known from the White Sea. While some of these taxa may represent regional variants of more widespread species, it is likely that all of these taxa occur elsewhere. This raises issues of where these species resided during glacial periods since it is unlikely that they have evolved in situ in the limited time since glacial retreat. The harsh environmental conditions of the Barents Sea would suggest limited exchange of species between the White Sea and temperate areas of the North Atlantic. Regardless, the relationships of populations of species in the White Sea to those elsewhere in the North Atlantic, North Pacific and Arctic Oceans should provide major insight into the evolutionary history of many species complexes and the post-glacial patterns of colonization of the upper latitudes of the Northern Hemisphere (c.f., Zinova 1950, Luning 1990, Vozzhinskaya and Luchina 1995).
One of the more curious aspects of the intertidal vegetation is the presence of several flowering plants intermingled with F. vesiculosus. While in many cold temperate areas one would expect to observe this in salt marshes, in the Keret Archipelago this is a main feature of rocky intertidal sites where Triglochin maritima is common in the mid to high intertidal zone and often grows well below the upper limit of F. vesiculosus. A second flowering plant, Zostera nana is common on muddy shores with low salinity and extensive patches of this species may have thousands of individuals.
While there are few species, the variation in some species is remarkable. In Fucus vesiculosus there are small, dark brown thalli on brackish shores to the more typically sized and pigmented forms in marine intertidal situations, to subtidal forms upwards of 1 m in length. This form variation is reminiscent of the west coast of North America where a single species defined on molecular criteria has evolved morphologically into a variety of niches (Kucera and Saunders 2008).
While the seashores are superficially lush with dense beds of Fucus vesiculosus and Ascophyllum nodosum, and these species are estimated to be responsible for almost 1% of total White Sea primary production (Berger 2009), careful searching is required to find epiphytic and endophytic taxa. There are epiphytic species, however, the species richness that one might expect on similarly lush shores is absent. Even in mid-summer, drift fronds of Saccharina latissima were almost clean of macroalgae, although elsewhere a more diverse community develops (e.g., Mikhaylova and Shtrik 2007). While there are extensive beds of Zostera marina, the leaves at the height of summer during August were also largely devoid of epiphytic algae apart from diatoms and extensive free-floating skeins of Cladophora glomerata in which they become entangled. This may be a consequence of the high freshwater inflow into the system to which some marine plants have become adapted.
Over 150 species names have been used for marine algae from the White Sea (Garbary unpublished data). Even though the identification of many of these species is uncertain (Mikhaylova personal communication), it would represent an equivalent assemblage of macroalgae to that reported for the Canadian Arctic including the temperate Hudson and James Bay (Lee 1980, Mathieson et al. 2010). The total species number is still considerably less than the Canadian Arctic, an area with a much harsher climate (Lee 1980, Wilce 1990, 1994). Although a complete inventory of White Sea algae requires more investigation, our geographically, temporally and depth limited study has found seven new records for the White Sea which represents an increase of about 7% to the flora. This suggests that as further investigations are made, that many additional species will be added. Regardless, the White Sea would seem to be species poor, given favorable climatic and oceanographic conditions and proximity to Western PopulaEurope. The isolated position of the White Sea resulting from climatic conditions and sea ice in the Barents Sea (e.g., Luning 1990) may have limited colonization into a fundamentally temperate water body. However, the migratory route across northern Europe was not always blocked, even during the Holocene. During the Hypsithermal (ca. 6000 BP) forests reached the northern shorelines of the Kola Peninsula (Kremenetski et al. 1997, MacDonald et al. 2000), and a migratory path from northern European species would have been open. The similarity in species composition between the White Sea and Western Europe may have had its origin during a Hypsithermal migration. With additional climate warming and reductions in coastal sea ice in the Barents Sea, we predict a greater influx of European algal species into the temperate White Sea.
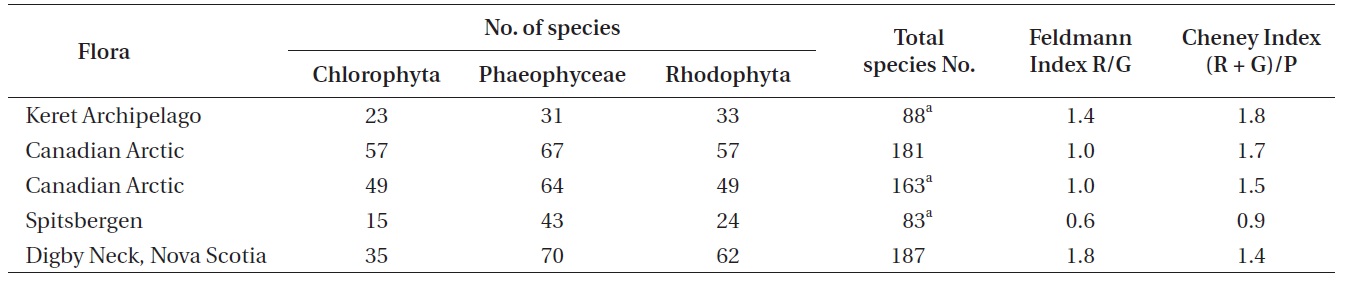
The Feldmann (1937) and Cheney (1977) indices have been used extensively as a general measure of the arctic to tropical nature of floras (see discussion of these metrics in Garbary 1987). Using the revised species totals from this study one can calculate the indices for the Keret Archipelago and other subarctic areas (Table 3). While the species richness is comparatively low and similar to Spitsbergen (Fredriksen and Kile 2012) the Feldmann and Cheney indices are at least 100% greater for the Keret Archipelago. The values for the Keret flora is even higher than that of Digby Neck in Nova Scotia, a clearly temperate climatic area that has at least double the species richness in an equivalent geographic area as the Keret Archipelago. The low species richness confirms the arctic nature of the flora, but the taxonomic distribution of the species is more consistent with a temperate flora. Thus, the biodiversity of the Keret Archipelago is contrary to the appearance of its intertidal flora with its lush beds of fucoid algae.