The genus Grateloupia was established by C. Agardh (1822) based on G. filicina (J. V. Lamouroux) C. Agardh. Grateloupia is distinguished by Grateloupia-type auxiliary cell ampullae with a single primary ampullar filament and two or three 7- to 13-celled secondary ampullar filaments of a conical out line (Chiang 1970, Kawaguchi 1989, Wang et al. 2000). Recent studies combining morphological and molecular data have resulted in several taxonomic changes. Pachymeniopsis Y. Yamada, Prionitis J. Agardh, Dermocorynus P. L. Crouan & H. M. Crouan, and Phyllymenia J. Agardh have been merged into Grateloupia (Kawaguchi 1997, Wang et al. 2001, De Clerck et al. 2005b, Wilkes et al. 2005) while G. intestinalis (Harvey) Setchell moved to Glaphyrosiphon Hommersand & Leister (Hommersand et al. 2010).
Grateloupia elata (Okamura) S. Kawaguchi & H. W. Wang was described based on specimens under the name Prionitis elata Okamura (type locality: Shirahama, Chiba Prefecture, located at the Pacific side of Japan). In Korea, Cotton (1906) reported this species (as Prionitis elata) for the first time at Wonsan on the northeast coast of the Korean peninsula. Afterwards, G. elata was reported between Busan and Gangneung on the east coast of Korea and Gapado on Jeju Island (Lee and Kang 2001).
Grateloupia cornea Okamura (1913) was transferred to the genus Carpopeltis and then changed to Prionitis cornea (Okamura) E. Y. Dawson (Dawson 1958). However, Wang et al. (2001) reinstated the original name, Grateloupia cornea, when they merged Prionitis into Grateloupia. In Korea, Kang (1966) reported this species on the east to south coasts and on Jeju Island. Subsequently, this species has been reported in many floristic reports (Lee and Kang 2001).
The taxonomy of the genus Grateloupia remains confused due to the low taxonomic utility of vegetative morphological characteristics, such as surface morphology, thallus habit and texture, and frequency of proliferations (Wang et al. 2001, De Clerk et al. 2005a, Wilkes et al. 2005). Recent studies show that the rbcL gene is a suitable marker for taxonomic study of Grateloupia (Wang et al.
2000, Faye et al. 2004, Lee et al. 2009, Yang et al. 2013). In the present study, we analyzed rbcL from unidentified specimens in Korea as well as other species and observed morphological characters. As a result, we describe Grateloupia jejuensis sp. nov. and discuss the phylogenetic relationships of G. jejuensis with the other species of the genus.
In total, 38 specimens of 13 species in the Halymeniaceae were collected from Korea, China, Japan, Russia, and USA. Field observation and collections of Grateloupia jejuensis were made at intertidal zone of 12 locations around Korea and Japan from 2009 to 2012.
Specimens were sectioned with a freezing microtome (FX-801; Yamato Kohki Industrial Co. Ltd., Tokyo, Japan). Photographs were taken with a DP-71 camera (Olympus, Tokyo, Japan) attached to a microscope (BX 51; Olympus). Voucher specimens are housed at the herbarium of Chungnam National University, Daejeon, Korea (CNUK).
We analyzed 84 sequences representing 48 Halymeniaceae species, including 38 new sequences and 46 GenBank sequences. Information for specimens, their collection data, and GenBank accession numbers of rbcL sequences are given in Table 1. DNA extraction, PCR amplification, and sequencing follow the procedures in Boo et al. (2010). For amplification and sequencing reaction of the rbcL gene, specific primer pairs were rbcLF145- rbcLR898 and rbcLF762- rbcLR1442 (Kim et al. 2010). Electropherogram outputs from each sample were edited using Chromas version 1.45 (http://www.technelysium.com.au/chromas.html). Eighty-nine rbcL sequences were collated and aligned using Se-Al (version 2.0a11), as per Rambaut (2002). Maximum likelihood analyses were conducted using RAxML (Stamatakis 2006) with the GTR + Γ evolutionary model. We performed 200 independent tree inferences using the -# option with default ?I (automatically optimized SPR rearrangement) and ?c (25 distinct rate categories) option in the program to identify the best tree. To generate bootstrap values for the best phylogeny, we used 1,000 replications under the same program with the same model settings.
Thallus solitariam aut caespitosa, applanatis et dichotomis calami, saepe flabellatae in adumbration, apice cum terminus in obtunsus aut bifida, cartilaginous in textum. Cortex cum 7-8 compactus corticales cellulis, sphaericus in intimus cellulis et angulata superficies cellulis. Medulla cum parvos sphaericus cellulis, 5-7 μm in diametros. Tetrasporic sporophylls, tenuis et parvos, oblongae, acuminatus ad basin, seriatum per duo marginibus. Tetrasporangi is cruciatis dividuus, plasmatio ex corticales cellam iacuit in the proliferous ramulis.
Thallus solitary or caespitose, flattened and dichotomous branches, often flabellate in outline, apex with ending in blunt or bifida, cartilaginous in texture. Cortex with 7-8 compact cortical cells, spherical in innermost cells and angulated surface cells. Medulla with small spherical cells, 5-7 μm in diameter. Tetrasporic sporophylls, thin and small, oblong, tapering at base, seriated along both margins. Tetrasporangia cruciately divided, forming from cortical cell layer in the proliferous branchlets.
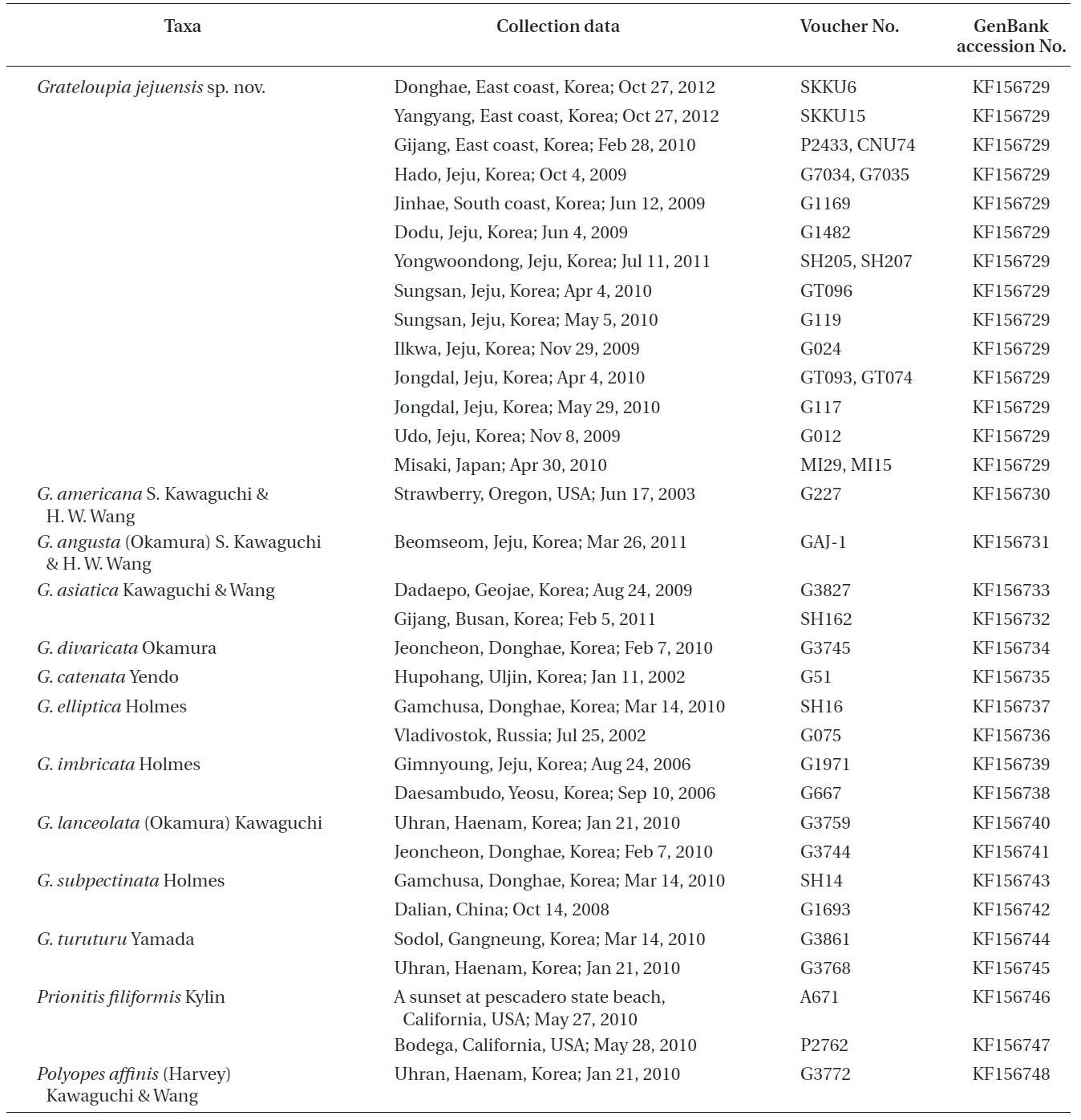
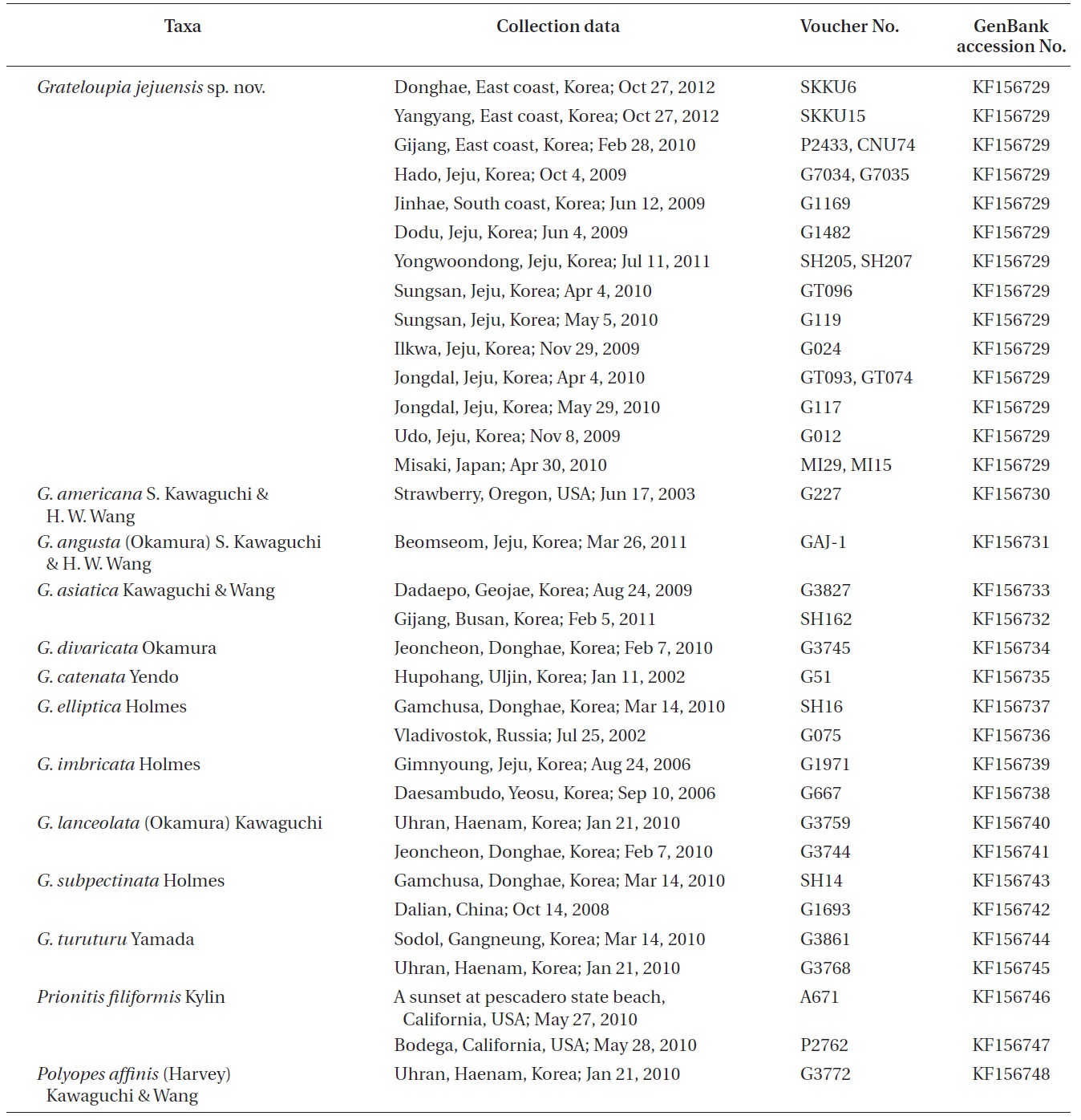
Holotype. CNU040218, intertidal zone of Hado (33°31? N 126°54? E), Jeju, Korea, Oct 4, 2009 (Fig. 1A), Herbarium of Chungnam National University (CNUK), Daejeon, Korea. Isotypes: CNU040217, -19, -220-1, NIBRAL0000138259- 60.
Etymology. The specific epithet refers to Jeju Island, Korea, where the type was collected.
Distribution. Found from Gangneung on the east coast to Jinhae on the south coast, and from Ilkwari to Sungsan around Jeju Island. Grateloupia jejuensis was frequently found throughout the year on rocks in tide pools and sheltered places in lower tidal zones. Specimens were collected in February, June, July, October, and December; tetrasporophytes were collected in May and October. Gametophytes were not found.
Korean name. 댓잎도박
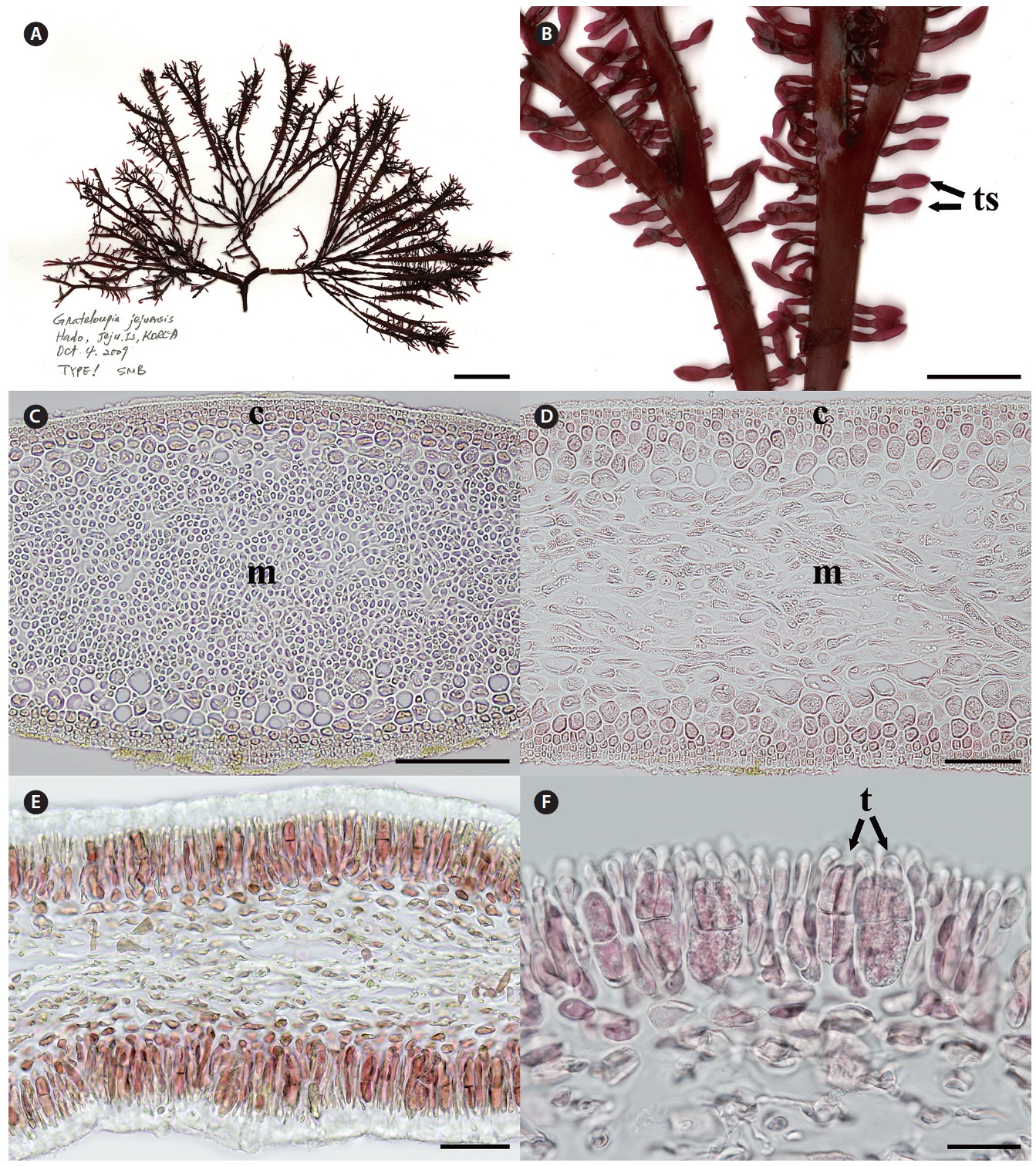
Morphology. Plants are solitary or caspitose, arising from discoid holdfast, and cartilaginous in texture. The thallus is dark purple to red in color and bright red in the upper portion. The thallus is 8-16 cm high, 0.2-0.4 cm wide, and 200-350 μm thick, with a short stipe (0.5-1 cm long). Branches are divided up to eight times dichotomously, and apex endings are blunt or bifid. The marginal branches produce proliferous branchlets that are often irregular and small (up to 5 mm long and 1 mm wide), scattered over the middle part of the thallus except for the stipe and basal part. The internal thallus is composed of compacted cortex and dense medulla. The cortex is 7-8 cell layers and is 5-20 μm in diameter. The outer cortex is composed of 3-4 layers with narrowly ellipsoidal cells, and the inner cortex is composed of 3-4 layers with irregular or rounded transparent cells. The medulla is composed of filamentous cells (5-7 μm in diameter). Tetrasporangia are formed from the cortical cell layer in the proliferous branchlets. The mature tetrasporangia are cruciately divided, narrowly ellipsoidal in shape, and 30-40 μm long by 10-15 μm wide.
Phylogeny of rbcL. 1,094-nucleotide portions of the rbcL gene were aligned for 84 sequences (38 new sequences),
[Fig. 1.] Grateloupia jejuensis S. Y. Kim, E. G. Han & S. M. Boo sp. nov. (A) Holotype (CNU040218, tetrasporophyte) of Grateloupia jejuensis collected from Hado, Jeju, Korea on Oct 4, 2009, deposited in the Herbarium of Chungnam National University (CNUK), Daejeon, Korea. (B) Close-up view of tetrasporic sporophylls (ts). (C) Cross section of thallus showing cortex (c) and medullar layers (m). (D) Longitudinal section of thallus showing cortex (c) and medullar layers (m). (E) Cross section of tetrasporic sporophylls. (F) Close-up view of a tetraspore (t). Scale bars represent: A, 2 cm; B, 5 mm; C, 100 μm; D & E, 50 μm; F, 20 μm.
representing 48 species of Halymeniaceae. All sequences of G. jejuensis from 12 sites in Korea and Japan were identical. The rbcL difference of G. jejuensis with Prionitis filiformis Kylin was 14-15 bp (1.28-1.37%), while G. jejuensis differed by 27-29 bp (2.28-2.45%) from G. elata and 63 bp (5.48%) from G. cornea.
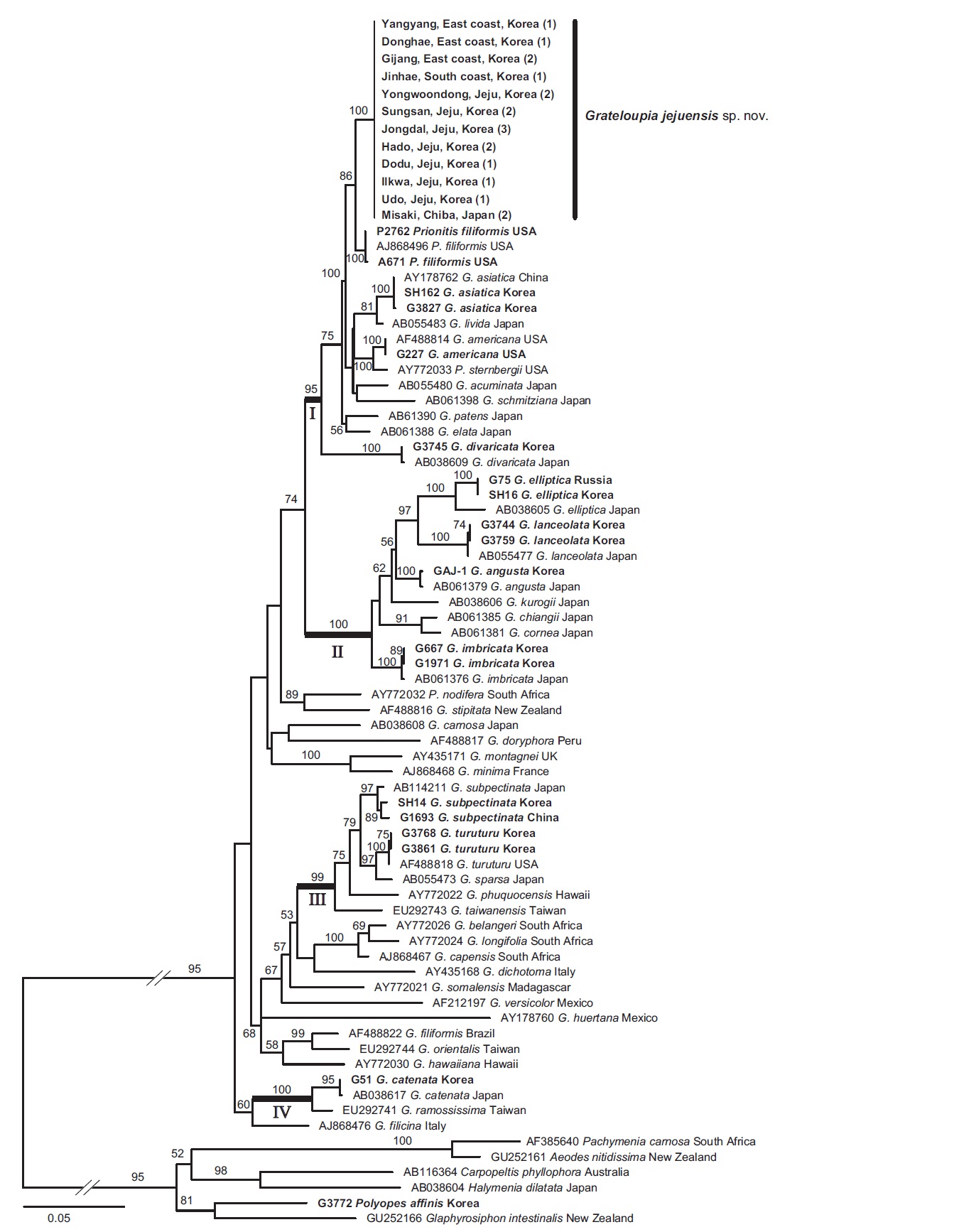
In the phylogenetic tree (Fig. 2), Grateloupia jejuensis was placed in a single clade and clearly separated from the other species of the genus. The sister species of G. jejuensis was Prionitis filiformis (86% for maximum likelihood). Grateloupia cornea was resolved as a sister taxon of G. chiangii from Japan with 91% bootstrap support. Grateloupia angusta shows sister relationships with G. elliptica and G. lanceolata.
The main finding of the present study is the discovery of a new species, Grateloupia jejuensis, in Korea based on rbcL sequence data and morphological evidence. It had previously been misidentified as G. elata (Lee 2008) or G. cornea (Lee 1987) because of similarity in cartilaginous texture, and erect, linear, compressed habit. In this context, the existence of G. elata and G. cornea in Korea may be questionable.
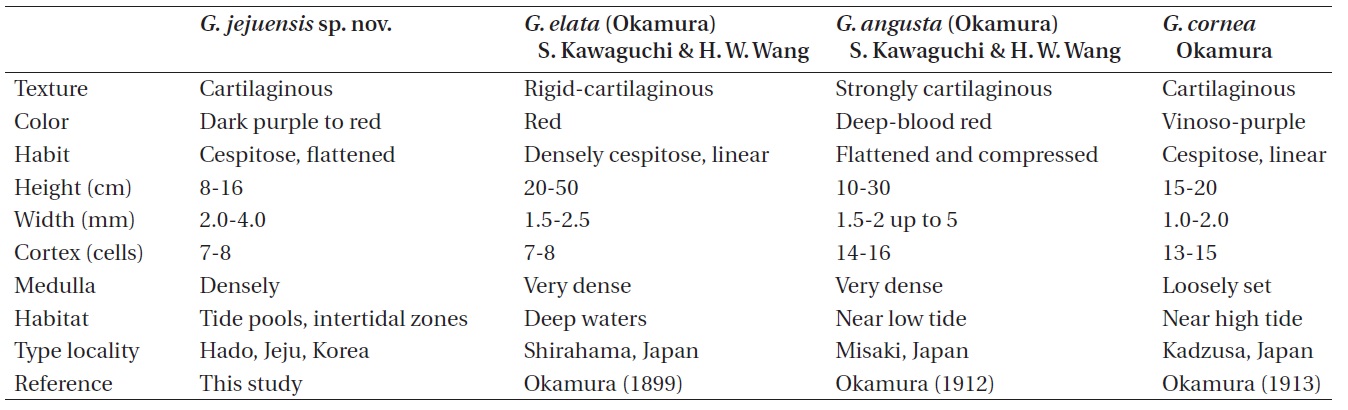
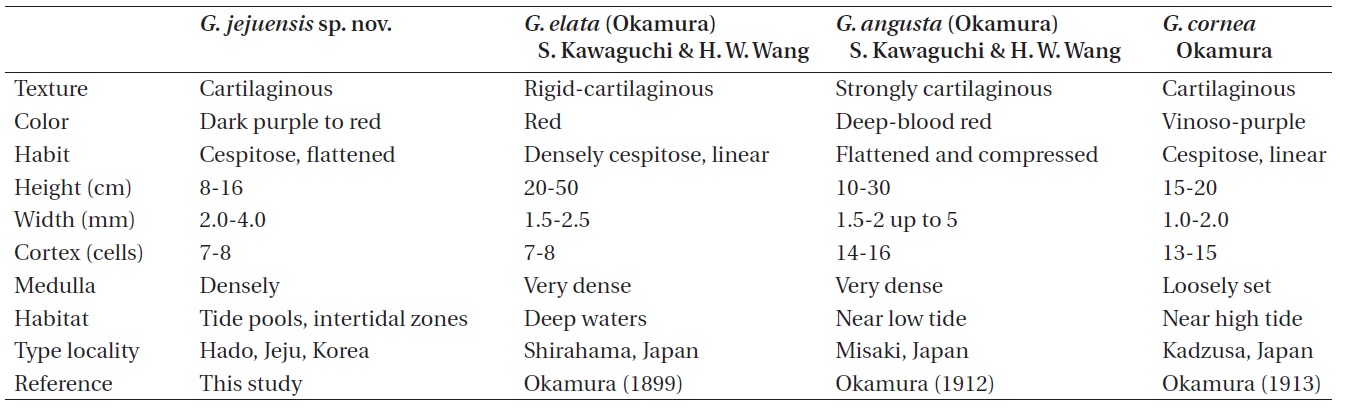
The comparative morphology of Grateloupia jejuensis with similar species is summarized in Table 2. Grateloupia elata is much larger in thallus size than G. jejuensis (20- 50 cm vs. 8-16 cm). Kawaguchi et al. (2001) reported that the difference in size is the one of main characteristics to distinguish G. asiatica from G. filicina (10-30 cm vs. 9-12 cm). The width of G. elata is less than that of G. jejuensis (1.5-2.5 mm vs. 2-4 mm). The difference in habitat is also a remarkable characteristic between the two species: G. elata
occurs in deep waters (Okamura 1899), whereas G. jejuensis usually occurs in tide pools and / or intertidal zone. Grateloupia cornea is also similar to G. jejuensis in having cartilaginous texture and linear habit. However, G. jejuensis has 7-8 cortical cell layers, while G. cornea has 13-15 cortical cell layers. The structure of medullary cells of G. jejuensis is more densely constructed than those of G. cornea.
Okamura (1899) stated that G. elata resembles G. angusta (Okamura) S. Kawaguchi & H. W. Wang in structure, habit, and substance, but differed in fruit-bearing (tetraspores and cystocarps) portion and cells of the intermediated layer. G. angusta is distinguished from G. jejuensis by composition of medulla and number of cortical cells. G. jejuensis has a densely composed medulla with 7-8 cortical cells, while G. angusta has a very densely composed medulla with 14-16 cortical cells.
Wang et al. (2001) merged Prionitis to Grateloupia; however, the formal proposals were not for all species of Prionitis. The genus Prionitis is still used in the Halymeniaceae. In our rbcL tree, P. filiformis was the sister of G. jejuensis and grouped together with all other Grateloupia species with high bootstrap support. It is therefore suggested that P. filiformis can be transferred to Grateloupia. Before taxonomic revision, a more detailed morphological study of P. filiformis is needed.
In the phylogenetic tree (Fig. 2), ten Korean species were grouped into four different subclades with strong bootstrap support. Grateloupia jejuensis was included in subclade I. Prionitis filiformis, P. sternbergii (C. Agardh) J. Agardh, Grateloupia americana from the northeast Pacific and Grateloupia acuminata Holmes, G. asiatica, G. divaricata, G. elata, G. jejuensis, G. livida (Harvey) Yamada, G. patens (Okamura) S. Kawaguchi & H. W. Wang, G. schmitziana (Okamura) S. Kawaguchi & H. W. Wang from
the northwest Pacific, were mixed within this subclade, suggesting that many species were likely to have diverged in the north Pacific Ocean. Grateloupia cornea and G. angusta formed subclade II. Given the morphological similarity to G. jejuensis, the habit similarity is a result of convergence. This result is consistent with previous studies (Wang et al. 2001, De Clerk et al. 2005a, Wilkes et al. 2005, Lee et al. 2009). Thallus habits are homoplasious within Grateloupia; thus, additional taxon sampling will provide a better understanding of phylogenetic relationships of the species.