The development of natural drugs with antidepressant effects is important and needed. This study was performed to investigate the antidepressant-like effects of the distilled water extract of Portulaca oleracea L. (POL) in a mouse model and to investigate the role of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors in producing these antidepressant-like effects.
The forced swim test (FST) and tail suspension test (TST) were used to investigate the behavioral anti-depressive-like effects of POL in mice. Additional behavioral experiments with 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3- dione, an AMPA receptor antagonist, were undertaken to determine the involvement of the antidepressant-like properties of POL in AMPA receptor throughput.
Oral administration of the POL extract (100 mg/kg) 1 h prior to testing significantly reduced the immobility times in the FST and TST. The antidepressant-like effects of the POL extract were not increased in a dose-dependent manner. Pre-treatment with NBQX significantly attenuated the reduction in immobility time induced by the POL extract in the FST.
The distilled water extract of POL has antidepressant-like effects, which may be related to AMPA receptor. Pre-treatment with NBQX significantly attenuates the reduction in immobility time induced by the POL extract in the FST.
Depression is one of the main mood disoder that affect mind, physical health, and behavior. It is thought to be caused by disturbance of monoamine neurotransmitters in the central nervous system (CNS). It was predicted as the second most common disease in 2020 by World Health Organization (WHO)1).
Depression may be mutually influenced by biological, genetic, social, and psychological factors. The biological etiology of depression involves the induction mediated by a biochemical imbalance of brain neurotransmitter. This knowledge has spurred drug development2-4). Antidepressants currently in use include serotonin reuptake inhibitor (SSRI), serotonin-norepinephrine reuptake inhibitor (SNRI), norepinephrine-dopamine reuptake inhibitor (NDRI)5-11). Although most of the antidepressant medicines can ease depressive symptoms, they can also induce various unexpected side effects, and 30% of depressive patients do not respond properly to initial treatment12). SSRI side effects are less than those resulting from the use of tricyclic antidepressant (TCA) and monoamine oxidase inhibitors (MAOi). However, the side effects of SSRI are serious and include gastrointestinal problems, CNS excitement, and sexual dysfunction13).
Alleviating these side effects is of paramount importance in the development of new antidepressant drugs. The possible development options include natural products14). There is ample precedent for this, as many kinds of herbs have been used in traditional medicines to treat stress, sadness, anxiety, and depression15).
POL has an anxiolytic-like effect, as apparent from the elevated plus-maze test (EPM) in mice. This effect is mediated by the gamma-aminobutyric acid (GABA)ergic nervous system. This effect that was produced by a 70% ethanol extract of POL was blocked by an intra-peritoneal (i.p.) administration of 10 mg/kg of flumazenil, the GABAa antagonist, but not by the i.p. administration of the 0.3 mg/kg of the 5-hydroxytryptamine 1A (5-HT1A) antagonist WAY 100635. These results are evidence of the anxiolytic-like effect of POL, and suggest that this effect may be mediated through the GABAergic nervous system16).
POL contains flavonoids, coumarines, monoterpene glycoside, several nitrogenous compounds such as N-trans-feruloyltyramine, dopamine (DA), dopa (Dopamine), norepinephrine (NA), and several alkaloid compounds17). Furthermore, it has been demonstrated that NA and DA are the major bioactive components of POL18). Capillary electrophoresis has proven to be a useful, simple, and rapid technique for identification and determination of DA and NA in herbal medicines and POL plants19).
The collective foregoing results provide support for the view that POL might be effective in the treatment of depression. This study was performed to investigate the antidepressant-like effects of extract of POL in a behavioral despair animal model using the FST and TST. Both tests are commonly used to assess antidepressant activity20,21).
Existing antidepressants are hampered by their lag period. For monoaminergic-based antidepressants, this treatment lag can be several weeks in duration. This limitation results in appreciable morbidity and high risk of suicidal behavior, especially in the first 9 days after beginning antidepressant treatment22). Several previous studies aimed at identification and development more effective and well-tolerated drugs are underway, and are focused on glutamatergic-based therapeutics23-25).
The roles of
Given the neurotransmitter involvement of POL16,17,19), the present study investigated the possible involvement of POL with the AMPA receptor.
Male C57BL/6J mice (ORIENT BIO, Seoul, Korea) weighing 20∼22 g, were used for behavioral and biochemical experiments. The mice were housed in acrylic cages (22×27×12 cm) with free access to water and food under an artificial 12-h light/dark cycle (lights on at 7:00 A.M. and off at 7:00 P.M.), at a constant temperature (22±2℃) and humidity (40±10%). Mice were housed in the departmental room for 1 week before testing to ensure adaptation to the new environment. All of the behavioral experiments were performed between 9:00 and 15:00. The present study was performed in accordance with the Kyung-Hee University Laboratory Animal Research Center guidelines for the care and use of laboratory animals.
POL was obtained from an oriental drug store (Jechon hanbang, Korea) and also from plants collected from Jechon, Korea, from May to July, 2011. The dried POL samples (600 g) were immersed in 2,500 ml of distilled water (D.W) and boiled at 100℃ for 1 h. The DW extract was collected. The extracts were filtered through 110 mm filter paper (Advantec Inc., Tokyo, Japan). This process was performed once more (100℃ reflux for 40 min). The filtrate was evaporated on a rotary evaporator under reduced pressure and temperature, and freezing-dried to yield about 39.7% (w/w) of the extract. The crude extracts were completely dissolved in distilled water immediately before use and administered to the mice orally (100, 500, and 1,000 mg/kg, p.o.). For each drug treatment, control mice received the respective vehicle alone.
The FST was performed by an established process 31). The apparatus consisted of a transparent Plexiglas cylinder (height 35 cm, width 20 cm) filled to a 20 cm depth with water at room temperature. Each mouse was kept in each cylinder of water between 23 and 25℃. The duration of immobility during the last 4 min of the 6-min test were measured. Mobility was set as any movement beyond that needed to keep the head above water. At the end of the trial session, mice were transferred from the water, dried with a paper towel, and put back in their home cages. To investigate dose-dependent antidepressant-like effects of the DW extract of POL, POL extracts (100, 500, or 1,000 mg/kg) or DW were administered p.o. 1 h before the FST. Six to nine mice were used for each group.
The TST was performed as described previously6) with modifications. Mice were hung by the tail from a metal rod using adhesive tape. The rod was fixed 45 cm above the surface of a table in a sound-blocked room. Each mouse was positioned at least 15 cm away from the nearest object. The test sessions were recorded for 6 min, and the immobility time was determined by one observer. Mobility was defined as movement of the hind legs. To investigate dose-dependent antidepressant-like effects of the DW extract of POL, POL extracts (100, 500, or 1,000 mg/kg) or DW were administered p.o. 1 h before the test. Five to seven mice were used for each group.
Statistical analyses were executed using SPSS ver. 18 computer software (SPSS Inc., USA). Data were analyzed using one-way analysis of variance (ANOVA). Tukey HSD and LSD post hoc test were utilized to compare significant ANOVA results. Results are presented as the mean±SEM. All statistics were 2-tailed. Statistical significance was set at p<0.05.
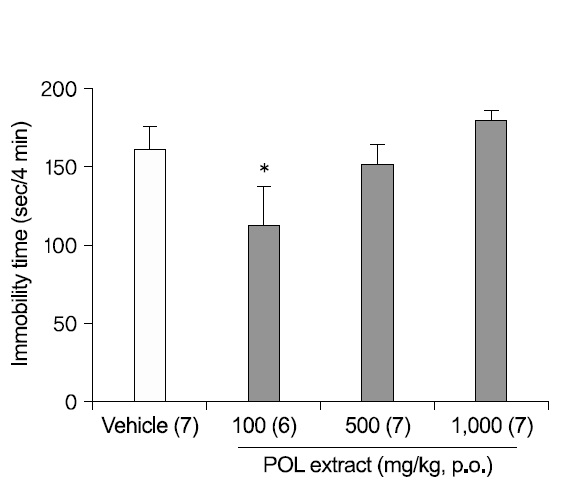
1. Antidepressant-like effects of POL extract in the FST
To study the dose-dependent antidepressant-like effects of POL DW extract, the FST was used. Mice were administered with DW or POL extract (100, 500, or 1,000 mg/kg) 1h before the test (Fig. 1). An oral administration of POL extract (100 mg/kg) significantly decreased the immobility times in the FST (p<0.05), although extract concentrations of 500 mg/kg and 1,000 mg/kg did not decrease significantly the immobility times. The results indicated that the antidepressant-like effect was not dose-dependent.
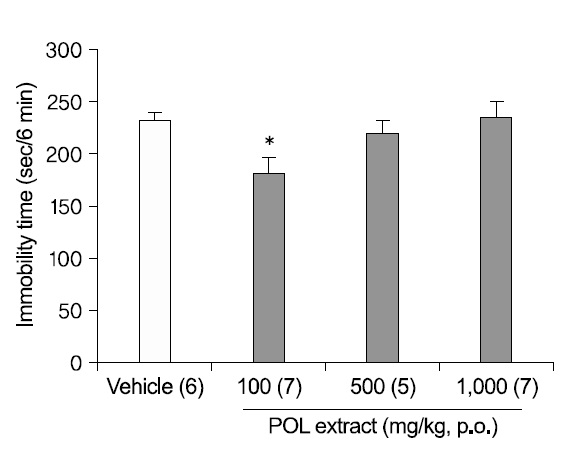
2. Antidepressant-like effects of POL extract in the TST
Mice were fed with DW or POL extract (100, 500, and 1,000 mg/kg) 1 h before the test (Fig. 2). An oral administration of 100 mg/kg POL extract significantly decreased the immobility times in the TST (p<0.05), although the results at 500 mg/kg and 1,000 mg/kg were not significant, meaning that the antidepressant-like effect of POL extract did not ascend dose-dependently.
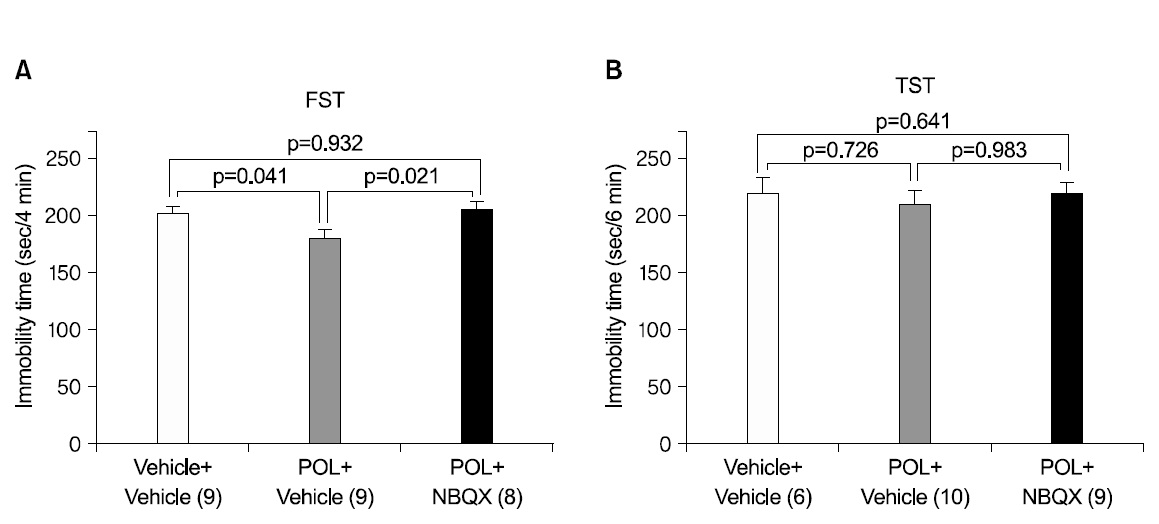
3. Effects of AMPA receptor blockade on the antidepressant-like effects of POL extract in the FST
We administered 100 mg/kg POL extract or DW to mice 1 h before the FST, followed by i.p. injection of the AMPA receptor antagonist NBQX or vehicle 40 min prior to testing. We selected a dose of 10 mg/kg of NBQX because a dose in this range has been demonstrated to decrease AMPA-induced seizures in mice11). NBQX pretreatment significantly attenuated the reduction in immobility time induced by POL extract in the FST (Fig. 3A, p <0.05). Post hoc analysis revealed a significant effect of POL extract alone compared to the each of the other two groups (both p<0.05) and revealed no other significant differences. But, the antidepressant- like effect of the administration of 100 mg/kg POL extract in the TST were not significantly attenuated by NBQX (Fig. 3B, p>0.05). Post hoc analysis indicates that the POL extract did not significantly act when applied alone compared to each of the other two groups (p>0.05). We omitted the group treated by DW and NBQX, because, for the FST there was no significant effect of NBQX alone in changing the immobility time in comparison with vehicle treated mice in previous studies30,32-34).
In developed countries, particularly in the East Asia, an abundance of material possessions that are the outcome of robust economic growth can create an imbalance with spiritual culture life. The resulting anxiety and depression can fuel the pathophysiology of most cases of mental illness. Indeed, a survey revealed that mental disease is more prevalent in societies that have achieved high economic growth (Committee of Neuropsychiatrical textbook in national Korean Medicine).
Scientific advancements have enabled a better understanding of the nature of anxiety and depression at the molecular level. Nonetheless, this knowledge has not translated into an effective treatment, and the incidence and prevalence of depression have increased continuously. Typical antidepressants currently in use are SSRIs, SNRI, and 5 HT2 blockers7-11,35). When used for a long time, the drugs can cause problems such as tolerance, dependence, and side effects. So, it is important to develop the natural drugs to overcome these problems.
Portulaca oleracea L. (POL) is grown widely in Korea and China. Also, the various parts of POL have been applied in the clinical treatment of various diseases in Oriental medicine. It is widely used not only as an edible plant, but also used as a traditional oriental herbal drug. It has long been used to alleviate pain and swelling. It also used for the treatment of dysentery with blood stools, and externally for boils and sores, eczema, erysipelas, and the bites of snakes and insects. POL displays anti- bacterial, anti-viral, anti-caducity, and anti-diabetes activities, along with enhancement of immunity 20,32).
Several reports reported the isolation of alkaloids, coumarins, terpenoids (ginkgolide A, B, and C and bilobalide), flavonoids, and fatty acids from POL17,33,34). Interestingly, it reported that ginkgolides have a neuro-protective role against hypoxia- induced injury, which might be associated with their up-regulation of the expression of HIF-1-alpha in hypoxic neurons36). The ethanol extract from POL (EEPO) possesses notable anti-hypoxic activity, which might be related to promotion of the activity of the key enzymes in glycolysis and improving the level of ATP in hypoxic mice37). It et al., recently reported38) that POL possesses various effects on the nervous system, which include reduction in locomotor activity, anticonvulsant activity, inhibition of electrically stimulated contractions of nerve-muscle preparation, and muscle relaxant activity in conscious rats.
In addition, it was demonstrated that POL has an anxiolytic-like effect using an elevated plus maze test16). However, as far as we know, there have been no studies concerning the antidepressant effect of POL.
In this study, we utilized the FST and TST, given their validation as a measure of the efficacy of various antidepressants. The FST is a behavioral despair animal model, and has been shown to be the most valid predictor of antidepressant action39-41).
In the FST, oral administration (1 h before of test) of 100 mg/kg POL extract significantly reduced the immobility time. But, no significant effect was evident using 500, 1,000 mg/kg POL, indicating that the antidepressant-like effect of POL extract does not increase dose-dependently.
The TST was first described by Steru and his collegues 6). In the test, the tail-suspended mouse moves actively at first. But, if depressed due to stress, it is more immobile initially, followed by alternating periods of mobility and immobility. Antidepressants reduce this period of immobility, allowing the TST to be used to verify antidepressant activity. Like the FST, in the case of TST, an oral administration (1 h before testing) of 100 mg/kg POL extract significantly reduced the immobility time. But, no significant effect was evident at POL concentrations of 500, 1,000 mg/kg, indicating that the antidepressant-like effect of POL extract did not increase with increasing dose.
There are several possible explanations for the lack of a dose-dependent nature of POL. The 500, 1,000 mg/kg doses of POL may have been excessive and toxic/sedative. This explanation is consistent with the anxiolytic-like effect of POL via the GABAergic nervous system16).
Although multiple classes of medicines with monoamine-based mechanisms of action exist for the treatment of depression, many patients fail to achieve a sustained remission of depressive symptoms. The demands for improved pharmacotherapies for treatment-resistant depression suggest a great need for the development of new compounds with novel mechanisms of action such as glutamate transmission and related pathways42).
Modulation of the AMPA receptor subclass of glutamate receptors has been connected to the mechanism of action of antidepressant medications, as well as a target for future medications44-48). Recently, there has been demonstrated that the antidepressant- like effects of ketamine in the FST, as well as MK801, the NMDA antagonist and Ro25- 6981, the NR2B antagonist are prevented by pre-administration of NBQX28). Ketamine is of great interest because its’ efficacy includes rapid and relatively sustained antidepressant effects, and it is the only known drug or somatic treatment that results in such a dramatic and extended response with a single. However, adverse reactions may take place despite their acute and mostly chronic use, including neurotoxic effects, cognitive deficits, and psychotomimetic effects. Therefore, it is necessary and important to discover potential therapeutic agents derived from natural sources that exhibit antidepressant- like effect related to AMPA receptor activation.
Presently, pre-treatment with NBQX significantly attenuated the reduction in immobility time induced by POL extract in the FST. This indicates the possibility that antidepressant effect of POL is related with AMPA receptor activation.
But, pre-treatment with NBQX did not significantly attenuate the reduction in immobility time induced by POL extract in the TST. In our experiments, the experimental process was videotaped to accurately determine the immobility time. However, 3-4 mice (especially in the control group) could not be videotaped due to equipment problems. Thus, the differing numbers of mice in the experimental groups hamper the statistical significance of the results. Otherwise, it could mean that the antidepressant effect of POL might not be related with AMPA receptor activation. However, considering the problem of experimental course, it is difficult to affirm that the antidepressant effect of POL is never related with AMPA receptor activation.
Therefore, further studies are needed to establish those constituents that contribute to the antidepressant- like effects of POL extract. Additional animal studies, such as the Learned helplessness paradigm, are also required to demonstrate the rapid and sustained antidepressant-like effects of POL extract. To clarify the effect of POL on glutamatergic systems, a well-designed study is needed.
This study was carried out to investigate the antidepressant- like effects of DW extract of POL in behavioral despair models examined using FST and TST, and to discern whether the antidepressant-like properties of POL involve AMPA receptor throughput. An oral administration (1 h before testing) of 100 mg/kg POL extract significantly reduced the immobility times in the FST and TST (both p<.05). NBQX pretreatment (10 mg/kg, 40 min before testing, i.p.) significantly attenuated the reduction in immobility time induced by POL extract (100 mg/ kg, 1 h before testing, p.o.) in the FST. Post hoc analysis revealed a significant effect of POL extract alone compared to either of the other two groups (both p<.05) and no other significant differences. In conclusion, this study demonstrates that the DW extract of POL has antidepressant-like effects, and suggest that this effect might be related to the AMPA receptor.