In low-level laser therapy (LLLT), optical tissue clearing (OTC) has been considered as a useful method to enhance therapeutic efficacy by minimizing tissue turbidity, which limits the depth of light penetration and photon density in soft tissue [1,2]. Although various OTC methods, such as mechanical (positive pressure and stretching), chemical (hyperosmotic chemical agent [HCA]), and thermal (temperature) methods, have been studied to minimize tissue turbidity by reducing light scattering in soft tissue [3-6], the clinical applications have not been sufficiently studied to date.
Thermal methods are based on the mechanism of gel/liquidcrystalline phase transitions of all lipids in human subdermis [7,8], modifications of collagen fiber structure in human dermis [9], degradation of cellular components [10], and variation of the refractive index of intercellular fluid [11]. Khalil
Since Tuchin
Although many studies have been reported to evaluate the effect of laser pulse frequency (LPF) on LLLT [26], the efficacy of LPF [continuous wave (CW) or pulsed wave (PW)] on LLLT is still unclear, and there is no complete consensus for the mechanism of LLLT. However, it was generally confirmed that the PW laser may penetrate deeper into soft tissue as compared to the CW [26,27]. The PW from 2.5 to 10,000 Hz on LLLT was frequently used in the clinic due to the deep light penetration depth in soft tissue [26].
In this study, an optical tissue clearing laser probe (OTCLP) system was developed to utilize simultaneously or independently the three OTC methods of tissue temperature, LPF, and injection of glycerol. The OTCLP system was characterized by quantitatively evaluating OTC effects in
The studies of section 2.4.1 through 2.4.3 were separately investigated with different PSSs to obtain optimal OTC parameters. The study of section 2.4.4 has utilized the optimal OTC parameters which were sequentially applied to an identical PSS in order to minimize the variation of tissue conditions as a function of time.
2.2. Tissue Optical Clearing Laser Probe (OTCLP)
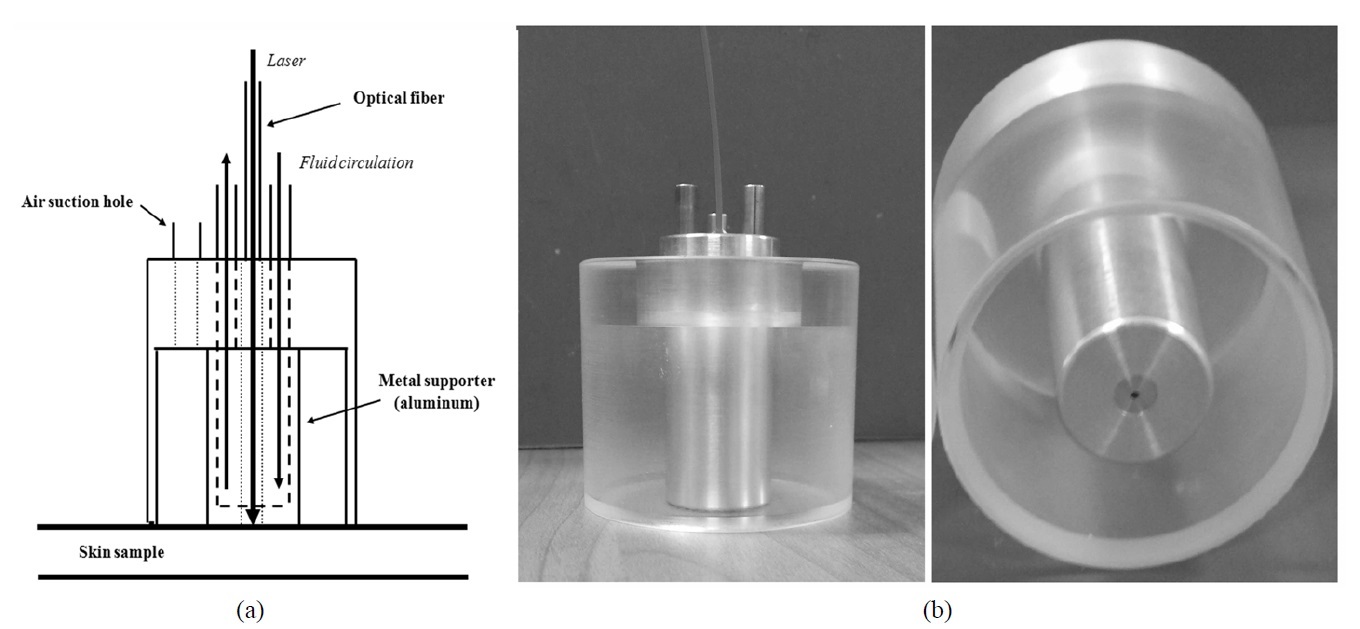
The OTCLP system was developed to increase the laser photon density (LPD) in soft tissue. Fig. 1(a) shows a schematic diagram of the OTCLP which consists of an air suction hole to supply negative compression for maintaining attachment, transparent acrylic body in order to observe skin deformation, and a metal supporter for both guiding an optical fiber and internal water circulation. The metal supporter was made from aluminum for high thermal conductivity. Fig. 1(b) shows the picture of the self-sustaining OTCLP, which can independently or simultaneously control tissue temperature, LPF, and negative compression.
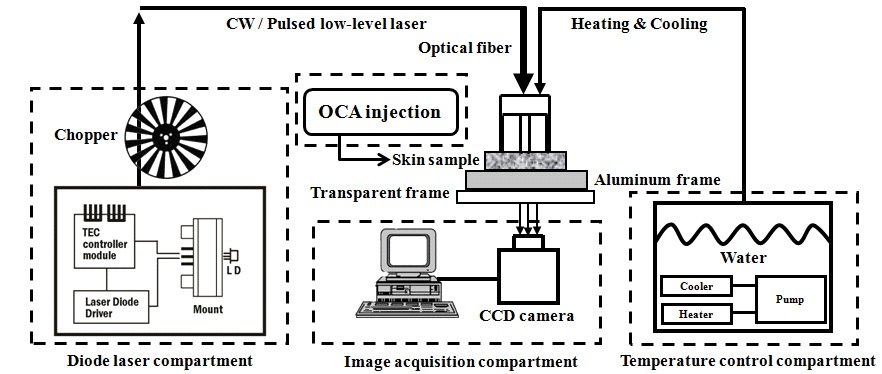
Figure 2 shows an experimental setup to obtain the diffusion images of the laser beam profile (LBP) which are transmitted PSSs. It consists of the compartments of diode laser, image acquisition, and temperature control.
The temperature control compartment has a refrigerating bath circulator (RW-0540G; Jeio-tech, Daejeon, Korea) to provide temperature-controlled water. The diode laser compartment consists of a 660 nm AlGaInP diode laser (HL6545MG; Thorlabs, Kansas, USA), diode laser mount (LDM21; Thorlabs, Kansas, USA) equipped with aspheric lenses (C280TME-B; A240TM-B; Thorlabs, Kansas, USA), TEC controller (TCM1000T; Thorlabs, Kansas, USA), laser diode driver (IP500; Thorlabs, Kansas, USA), and optical chopper (SR540; Stanford Research Systems, California, USA). This compartment controls the irradiation mode (PW or CW) of the low-level laser which is delivered to PSSs by a multi-mode optical fiber (FT400EMT; Thorlabs, Kansas, USA). Maximum laser power was adjusted to 10 mW at the distal end of the optical fiber in order to avoid light saturation in the LBP image. The image acquisition compartment consists of a CCD camera (XC-HR57; Sony, Tokyo, Japan) to obtain the LBP images of transmitted PSSs.
A double-integrating sphere system (Avasphere-30; Avantes, Apeldoorn, Netherlands) [31-33] which is equipped with a Quartz Tungsten-Halogen lamp source and a fiber optics spectrometer (USB4000; Ocean Optics, Florida, USA) was used to measure optical properties of PSSs as a function of tissue temperature. The data at 660 nm from the measured spectrum were used because this study used a 660 nm diode laser.
A combination and three independent methods were
applied to the PSSs to obtain LBP images: 1) as a function of tissue temperature ranging from 40 to 10℃ at 10℃ decrements; 2) as a function of the LPFs ranging from 5 to 30 Hz at 5 Hz increments; and 3) immediately after injection of 95% glycerol in the region of interest (ROI); and 4) by applying a combination method of the aforementioned three methods. The LBP image of 251×251 pixels was extracted and analyzed with a laboratory-built MATLAB program. The OTC effects of each method were quantitatively evaluated by computing the peak intensity (PI) and the total intensity (TI) which is defined as the intensity integration at full width at half maximum (FWHM) on LBP.
2.4.1. Tissue Temperature
LBPs were obtained as a function of tissue temperatures from 40 to 10℃ at 10℃ decrements. The LBP obtained at 40℃ was used as control data and the others as test data. The temperature of PSSs was controlled by circulating temperature-controlled water through the metal supporter of the OTCLP.
In order to investigate optical property variation due to tissue temperature, the PSSs were maintained at the temperature of interest in a refrigerating bath circulator before measurement, and then measurements were performed immediately after the PSSs were exposed to room temperature. Both transmittance and reflectance of PSSs were measured as a function of tissue temperature. Reduced scattering coefficients (μ's) [35] were computed with an Inverse-Adding Doubling program [35,36].
2.4.2. Laser Pulse Frequency (LPF)
LBP images of PSSs were obtained at 50 frames/sec as a function of LPF. CW and PW irradiation modes were used as control and test data, respectively. The LBP images with maximum PI were selected at each LPF and used for quantitative analysis of LBP.
2.4.3. 95% Glycerol Injection
95% glycerol was injected at a ROI where the laser was irradiated and LBP images were obtained immediately after glycerol injection. The PSSs with and without glycerol injection were used as control and test data, respectively.
2.4.4. Comparison of Independent and Combination Methods
The optimal OTC parameters for the combination method were derived from the best results of the three independent methods (section 2.4.1 through 2.4.3). The LBP for control data was first obtained at the tissue temperature of 40℃, and then independent and combination methods were sequentially applied to obtain the LBPs for test data as follows: 1) the optimal LPF laser was applied at the tissue temperature of 40℃; 2) 95% glycerol was injected and then the CW laser was applied at the tissue temperature of 40℃; 3) tissue temperature was decreased to optimal tissue temperature and then the CW laser was applied at the region without glycerol injection; and 4) the combination method was finally applied.
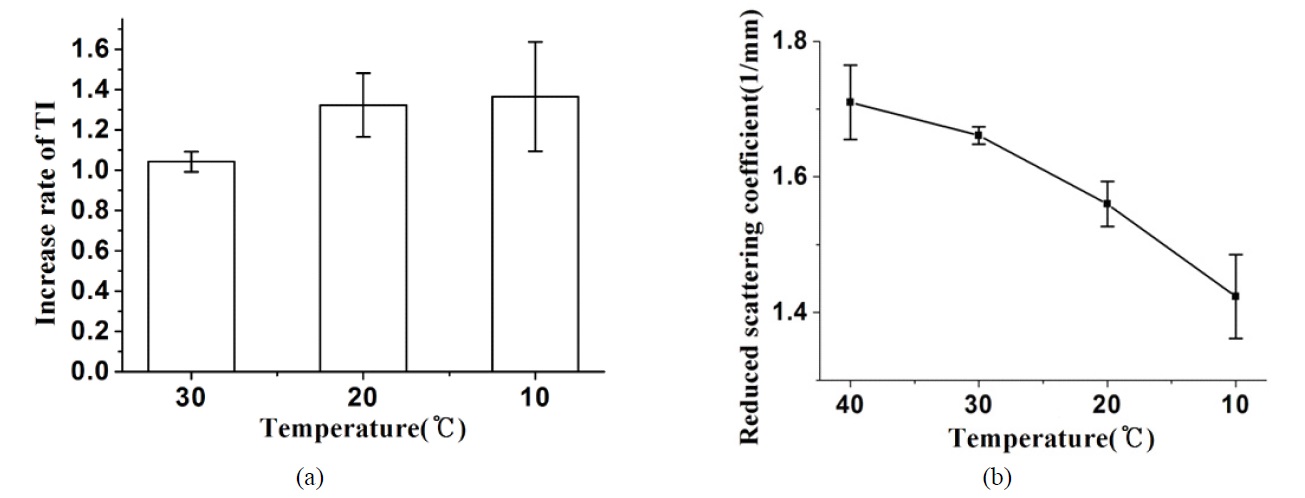
The laser intensity was increased by decreasing the temperature of PSSs, and the maximum and minimum laser intensities were observed at 10℃ and 40℃, respectively. Fig. 3(a) shows that average TI were 1.04-, 1.32-, and 1.37-fold higher at 30, 20, and 10℃ when compared to 40℃, respectively. Fig. 3(b) shows the μ's as a function of temperature that resulted in 1.71 mm-1 at 40℃, 1.66 mm-1 at 30℃, 1.56 mm-1 at 20℃ and 1.42 mm-1 at 10℃.
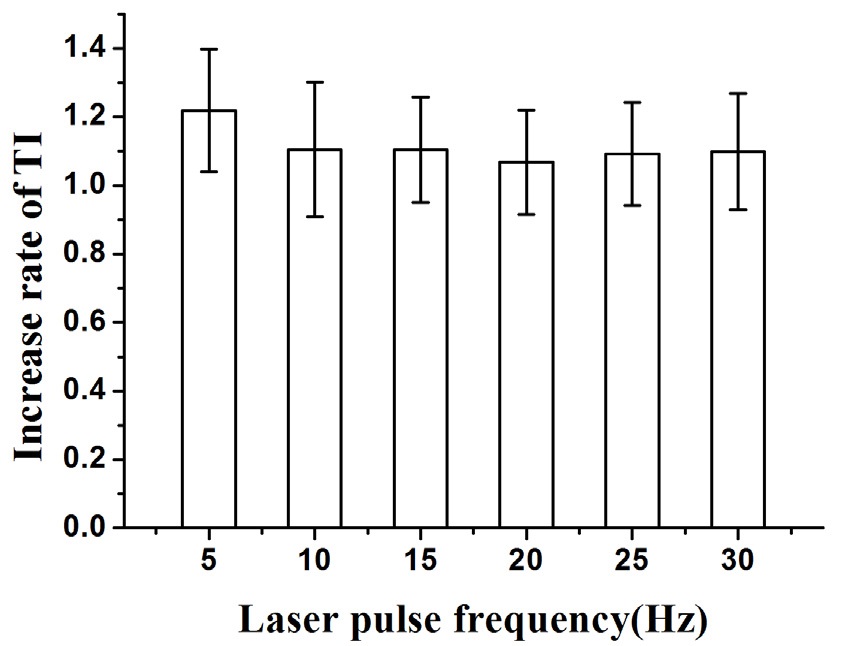
Figure 4 shows an average increase rate of TI at each LPF in which 5 Hz was determined to have the highest
increase rate of 1.22-fold. Other LPFs presented a negligible increase rate of 1.11-fold at 10 Hz, 1.10-fold at 15 Hz, 1.07-fold at 20 Hz, 1.09-fold at 25 Hz, and 1.10-fold at 30 Hz.
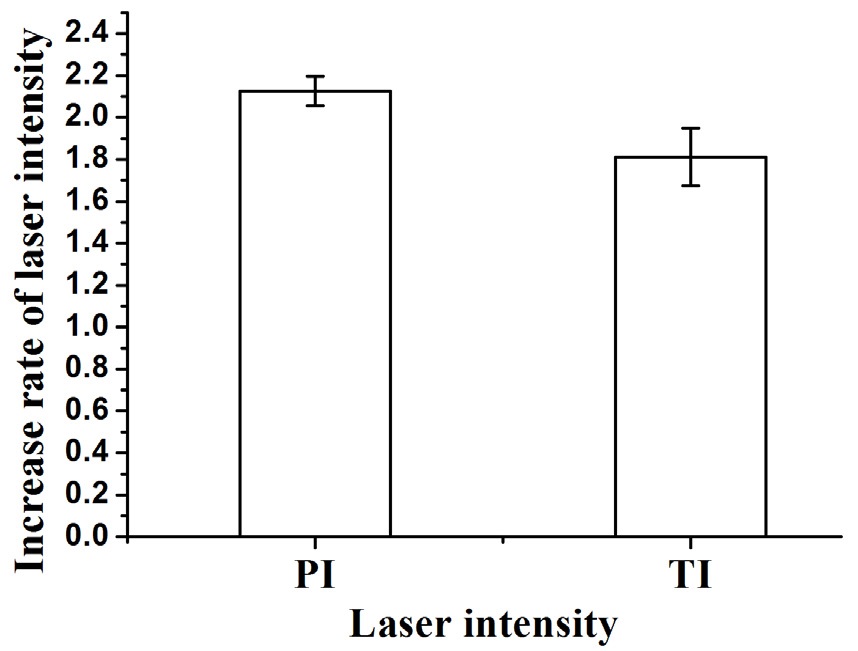
Figure 5 shows the increase rate of PI and TI in 95% glycerol injected PSSs that were used as test data while a non-injected PSS was used as control. The LBP of test data resulted in a remarkable enhancement of laser transmission. It showed that PI and TI were 2.13-fold and 1.81-fold higher in test than control data, respectively.
3.4. Comparison of Independent and Combination Methods
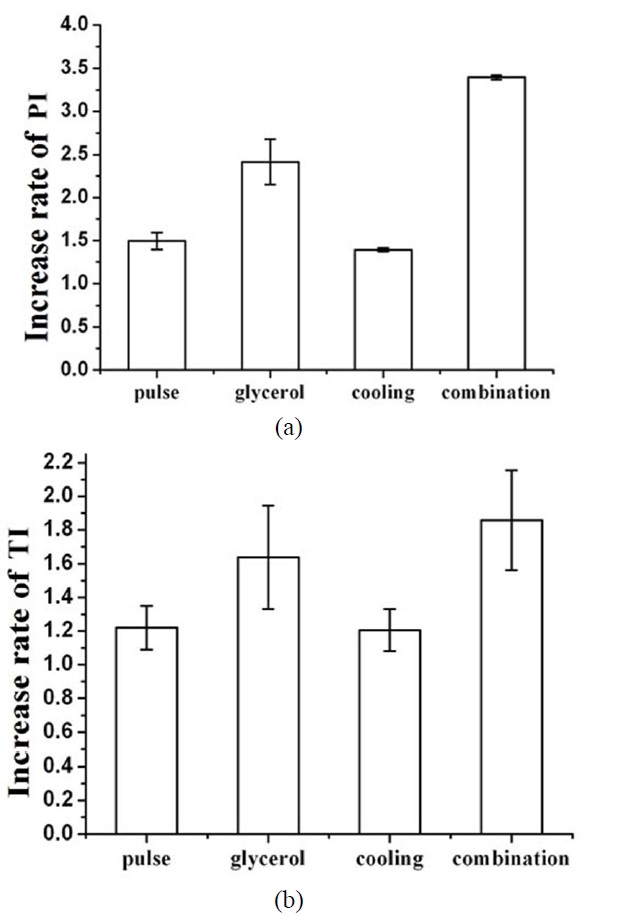
Figure 6 shows an increase rate of PI and TI for: (1) LPF of 5 Hz, (2) 95% glycerol injection, (3) tissue temperature of 10℃, and (4) a combination of the aforementioned three methods. Fig. 6(a) shows that the PI of the combination
method (3.40-fold) was higher than LPF (1.49-fold), glycerol injection (2.41-fold), and tissue cooling (1.39-fold) methods. Fig. 6(b) presents that the TI of the combination method
(1.86-fold) was also higher than LPF (1.22-fold), glycerol injection (1.64-fold), and tissue cooling (1.21-fold).
3.5. Comparison of Laser Beam Profiles
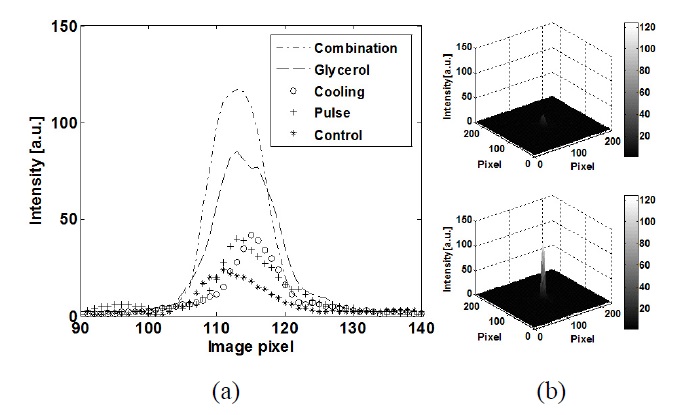
Figure 7(a) shows 2D LBPs of tissue cooling (10℃), LPF (5 Hz), 95% glycerol injection, the combination method, and the control. The 2D LBPs showed that OTC methods resulted in higher PI than the control, and the combination method resulted in relatively higher PI than the independent methods. Fig. 7(b) illustrates 3D LBPs of the control (upper) and the combination method (lower) and graphically presents a much greater OTC effect of the combination method.
LLLT has been primarily used in wound healing, tissue regeneration, pain and inflammation relief, prevention of tissue death, and mitigation of neurological degeneration [3]. However, tissue turbidity decreases the therapeutic efficacy of LLLT due to light scattering in soft tissue. In order to enhance tissue clarity, various OTC methods have been investigated [1,2]. Compression, tissue cooling, and HCA application were suggested as effective OTC methods to minimize light scattering in soft tissue. In this study, the OTCLP system was developed for independent or simultaneous application of OTC methods of tissue temperature, LPF, and glycerol injection in the ROI.
The OTC results from independent methods in sections 3.1 through 3.3 were slightly different from those in section 3.4 although an identical OTCLP system was used. For example, OTC effects at 10℃ were increased 1.37-fold in section 3.1 and 1.21-fold in section 3.4. For comparison of OTC effects (Figs. 6 and 7), the independent and combination methods used different PSSs as described in section 2.1. Therefore, such differences might be caused by different thicknesses and dehydration of PSSs and different residue of the fat layer which might affect the optical properties of PSSs.
Although the optical property of PSS, μa, was not presented here because its variation was negligible from 40℃ (0.0325 mm-1) to 10℃ (0.0431 mm-1), it showed an increasing pattern with decreasing temperature. Fig. 3 demonstrates that decreasing the tissue temperature resulted in decreasing the μ's and, therefore, increasing the PI and TI which means enhancement of photon density in the dermis. As described in previous studies, such results may be caused by the modification of collagen fiber structure, degradation of cellular components, and change of the refractive index of intercellular fluid [15-20].
A previous study described that a PW laser may have a deeper penetration depth compared to the CW laser [26]. In this study, the TIs of the PW laser increased more than about 10% compared to that of the CW laser (Fig. 4), but the increase rates of TI may be negligible because they are closely within the error bars. As a result, the penetration depth of the laser may not be strongly affected by the LPFs except for at 5 Hz which presented about 20% of the increase rate of TI in this study (Fig. 4). The clinical efficacy of LLLT may not depend on only the penetration depth of laser [26]. Therefore, the use of the CW laser or PW laser should be carefully determined depending on the therapeutic purposes.
Figure 5 shows the rapid OTC effect of locally injected glycerol. The LBP remarkably increased when compared to control. Skin dehydration and collagen dissociation may cause the reduction of light scattering by minimizing refractive index mismatching [15-17]. Recently, Yu
The combination method presented the greatest OTC efficacy compared to other methods (Fig. 6), and it was graphically confirmed with 3D LBPs (Fig. 7). The combination method was successfully integrated in the OTCLP system. Mechanical tissue compression, which is one of the effective OTC methods [6,38-41], was not integrated into the OTCLP system because of the structural difficulty of the OTCLP system. The combination method presented similar increase rate of PI when compared to mechanical compression [6] applied using a 40 mm probe diameter with a negative compression of 30kPa. It is expected that if the mechanical compression method [6] is integrated into the OTCLP system, the laser photon density may be further enhanced in deeper tissue.
Comparisons of OTC methods may be valuable but are not easy because of differences in experimental setups and analysis methods [3,5,6,9,11,12,32,33,37-40]. However, Izquierdo-Roman
In this study, the OTC efficacy was quantitatively analyzed and compared by manipulating OTC methods, such as tissue temperature, glycerol injection, and LPF in soft tissue. Independent OTC methods were integrated into an OTCLP system in order to enhance and optimize OTC efficacy by controlling OTC factors. In future study, if a mechanical compression method is added on the OTCLP system, the efficacy of OTC may be further enhanced. It is expected that the OTCLP system may be clinically used as a non-invasive tool in low-level laser therapy (for instant, pain, wound healing, and laser acupuncture) of deeper tissue due to the increase of photon density.