Phthalate esters (PEs) are a group of organic chemicals used as plasticizers to increase the flexibility and durability of plastics. The commercial compound di-2-ethylhexyl phthalate (DEHP) has various industrial uses. This compound has been identified in industrial wastewaters, fish, and other aquatic organisms (Scholz et al., 1997; Sonnenschein and Soto, 1998; Acey et al., 2002; Chang et al., 2005). The frequency of exposure to these compounds and their potential effects on the reproduction of aquatic animals has stimulated research into their environmental distribution, bioaccumulation, fate and mechanisms of action (Staples et al., 1997; Kim et al., 2002). These esters are also suspected to be endocrine disruptors and to mimic estrogen (Sonnenschein and Soto, 1998; Shioda and Wakabayashi, 2000; Tollefsen, 2002). As phthalic acidderived compounds, PEs have been shown to induce estrogen- receptor-mediated responses (Jobling et al., 1995). Stress caused by environmental variables can affect the endocrine system, which in turn, through bi-directional communication, can affect other intimately linked systems, such as the immune and nervous systems, and prevent the maintenance of homeostasis (Balm, 1997).
The immune systems of fish are similar to those of higher vertebrates (Nakanishi et al., 2002). The thymus, spleen and kidney (especially the head kidney) serve mainly as immune organs (Romano et al., 1997). A healthy immune system is essential to protect an organism from infections and diseases and to maintain homeostasis and general good health. Mucus secretions on the gill and digestive tract surfaces in fish act as a first defense and protect them from the invasion of microorganisms, because the surface of the fish body is exposed directly to viruses and bacteria in the surrounding water (Braun et al., 1990). Non-specific biodefense functions, including the phagocytic activity of leucocytes and other components such as lysozymes and complementary systems in fish respond to rapidly remove various xenobiotics, which are recognized as foreign bodies (Bennani et al., 1995).
Various chemicals are released and accumulate in the aquatic environment, and coastal areas become a ‘sink’ for many of them. These chemicals are linked to various adverse effects, including immunotoxicity, in fish (Kollner at al., 2002). However, little is known regarding the effects of chemicals on the immune system, such as the function of leucocytes in fish exposed to PEs
Rock bream,
The fish were exposed to DHEP in 0.5-ton fiberglass-reinforced plastic tanks; each treatment group comprised 20 fish. Each tank received a flow of 7 L/min with continuous aeration. DEHP was purchased from Sigma (St. Louis, MO, USA). DEHP was dissolved in sunflower seed oil (SSO) immediately before intraperitoneal injection. The fish were injected with doses of 200-, 400- and 800-mg DEHP/kg BW. The first injection was given 2 weeks after acclimatization and the second was given 1 week later. The control group was subjected to an identical regimen; however, they were injected with an equal volume of SSO. Blood and lymphoid tissue samples were taken for assessment of blood, spleen and head kidney parameters at 1 and 2 weeks post injection.
Leucocytes were isolated from the spleen and head kidney using a modification of the method described by Fatima et al. (2001). Single-cell suspensions were prepared by dissociating the lymphoid tissues, using a cell dissociation sieve-tissue grinder kit (Sigma), in L-15 medium supplemented with 0.1% fetal bovine serum (FBS), 1% streptomycin/penicillin solution (S/P; Gibco, Rockville, MD, USA) and 10 U/mL heparin (Sigma). The resulting suspensions were purified in Percoll (Sigma) density gradients (34/51%), centrifuged at 400
Cells collected from the interface were washed twice by centrifugation (1,000
>
Assay of phagocytic activity
Phagocytic activity was evaluated using a cell suspension, as described by Ahmad et al. (1998). An 0.1-mL aliquot with a cell density of 1 × 107/mL in L-15 medium was mixed with an equal volume of L-15 medium containing 20% FBS and 1 × 108/mL heat-treated (100℃ for 1 h) yeast cells (
PI = A × B
, where A = the percentage of phagocytes engulfing at least two yeast cells and B = the average number of yeast cells engulfed by phagocytosis-positive cells. PC is the mean percentage of cells that are engulfed (Bin-Hafeez et al., 2003).
The lysozyme assay was performed according to Ellis (1990), with some modifications. First, 0.2 mg/mL
The experiment was repeated three times. Statistical analysis of the results was performed using the SPSS/PC+ statistical package (SPSS Inc., Chicago, IL, USA). ANOVA and Duncan’s test for multiple comparisons were used to identify significant differences between the control and treatment groups (Duncan, 1955). The significance level was set at
>
Splenic and pronephric cellularity
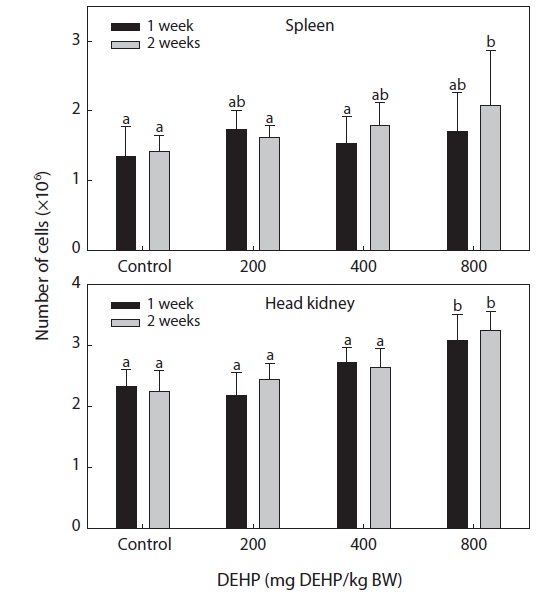
The total numbers of single cells in the spleen and head
kidney of fish exposed to different levels of DEHP are shown in Fig. 1. The number of cells in the head kidney was significantly higher in the fish exposed to 800-mg DEHP/kg than in the control group (
>
Phagocytic functional responses
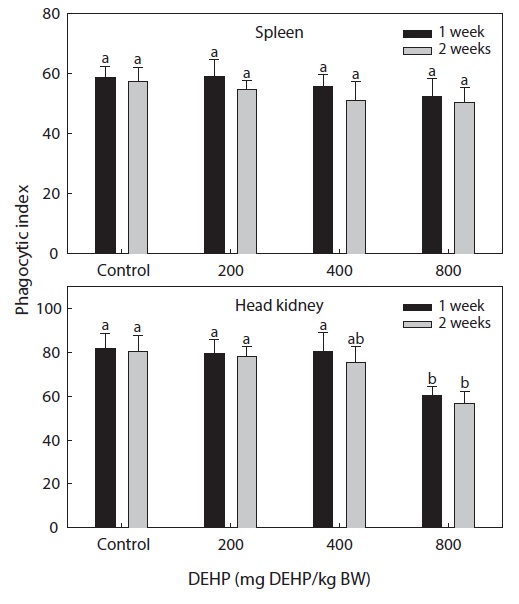
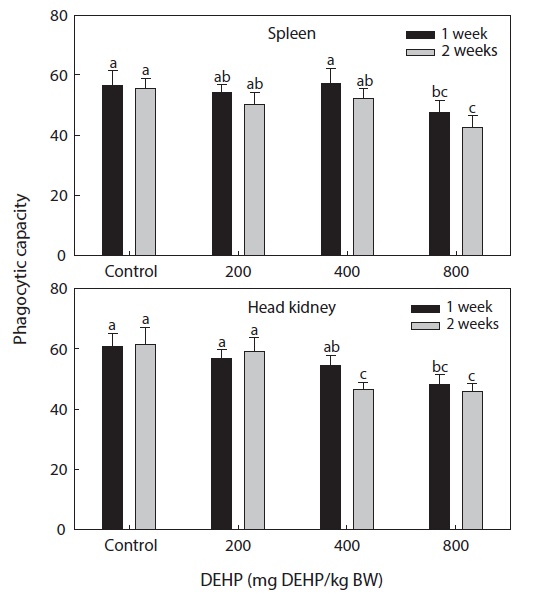
The dose-dependent effects on the nonspecific immune response and the phagocytic effects of DEHP in rock bream are illustrated in Figs. 2 and 3. The nonspecific immunity of rock bream decreased markedly upon application of 800-mg DEHP/kg BW, as indicated by the decreased PI in the head kidney (
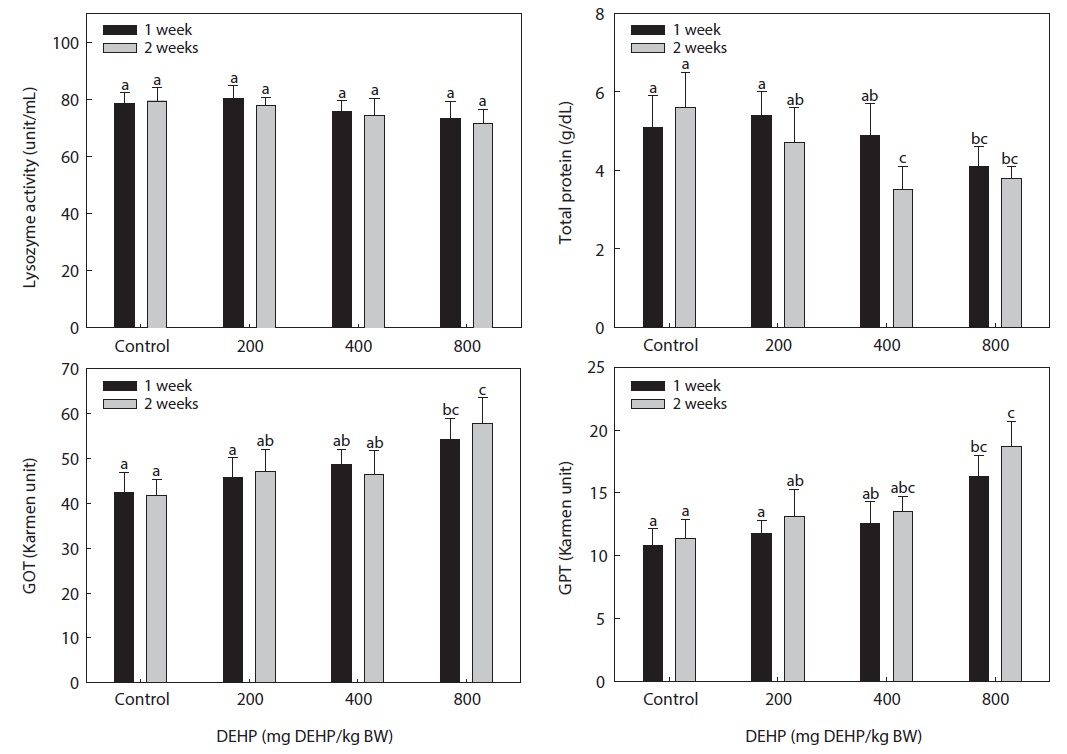
The lysozyme activity in the serum of rock bream exposed to DEHP is presented in Fig. 4. The DEHP decreased the serum lysozyme activity in rock bream in a dose-dependent manner; however, the activity in the treated groups was not significantly different from that in the carrier-injected control group.
The serum total protein concentration of rock bream treated with DEHP is shown in Fig. 4. The total protein concentration in rock bream exposed to ≥400 mg DEHP/kg BW DEHP was significantly suppressed compared to the control (
The immune systems of fish are similar to those of higher vertebrates, and tissues such as the thymus, spleen and kidney (especially the head kidney) function mainly as immune organs. Healthy immune systems are essential to prevent infection and diseases in fish and to maintain homeostasis and general good health. Markers of nonspecific immunity appear to be successful indicators of xenobiotic-induced stress in laboratory fish. Considerable evidence now supports links between environmental changes (including exposure to contaminants) and noninfectious diseases resulting from immunosuppression (Zelikoff et al., 2000; Fatima et al., 2001). The total number of leucocytes in fish hematopoietic organs is considered to be a more sensitive indicator of chemical toxicity than the activity of phagocytic cells contained within these organs (Hart et al., 1998). In lymphoid organs, high doses of DEHP can cause marked cellularity in the head kidney. The hypercellularity observed in the fish head kidney associated with treatment with 800-mg DEHP/kg BW is in agreement with the dose-dependent induction of thymocyte populations in mice by dioctyl phthalate (Dogra et al., 1993).
DEHP had an immunomodulatory effect on the phagocytic efficiency of rock bream leukocytes
The dose-dependent reduction in total protein and immunomodulation by DEHP suggested a disturbed physiological mechanism and a specific, receptor-mediated induction by PEs. The high GOT and GPT activity in rock bream treated with 800-mg DEHP/kg BW indicated a greater degree of hepatic dysfunction in immunosuppressed fish. The different responses of the immune system in spleen and head kidney leukocytes might have resulted from differences in the toxicity and distribution pattern of PEs in the organs of fish, as suggested by Menghi et al. (2002). Furthermore, the lack of significant changes in GOT and GPT activity also suggested normal hepatic function in fish treated with low DHEP concentrations. We conclude that exposure of the rock bream