Fish farming is acknowledged as an important and substantial part of the global fishing industry. The aquaculture industry in Korea has been developed and well sustained within the last few decades. Accordingly, aquaculture production has continued on an upward trend to reach 1,385,804 tons in 2007 (Yoon, 2008). Among the marine-culture commodities, the olive flounder
Fish are the most primitive vertebrates to possess an adaptive immune system. Hence, their innate immune system plays an instructive role in the acquired immune response and homeostasis for fundamental defense mechanisms (Magnadottir, 2006). The innate immune system is composed of three major parts: physical barriers, and humoral and molecular components. Therefore, several internal and external factors contribute to and influence the activity of the innate immune system. However, it is believed that food additives and immune-stimulants in fish feeds can enhance innate immunity. Increasing attention is being paid to the design and development of a fish feed that has a protective effect against oxidative stress and enhances immunity in fish species used in aquaculture.
Aquaculture is expected to positively influence fish nutrition and innate immunity to maximize survivability. Moreover, the effect of dietary nutrients on the juvenile olive flounder has been investigated recently (Cho, 2012). An increase in the quality of fish protein for human consumption is also expected. However, raw-fish-based moist pellet (MP) diets are commonly used in aquaculture systems in Korea, which may cause problems in flounder fish aquaculture, including disease outbreaks, water pollution and high production costs. Indeed, the pellets have been shown to have weak binding interactions with salt water and degenerate easily (Cha et al., 2006). A chitosan-based biopolymer material has therefore been used to coat MP, resulting in an improved water quality compared to the use of the non-coated MP diet (Cha et al., 2008).
Chitin (C8H13O5N) is a mucopolysaccharide polymer consisting of N-acetyl-D-glucosamine residues that are abundant in the shells of crustaceans and insects. Chitosan is derived from chitin by deacetylation and is manufactured commercially on a large scale for various biomedical applications. Chitin is the second most abundant biomass, after cellulose (Park and Kim, 2010). Dietary chitin and chitin-rich modulates support and enhance immune-stimulant activity when used as a supplement in the feed of many fish species (Ringø et al., 2012). Chitosan possesses excellent properties including low-toxicity, biocompatibility, low cost, and good handling properties (Ravi Kumar, 2000; Berger et al., 2004). Chitosan preparations have been determined to protect salmonids against bacterial disease (Anderson and Siwicki, 1994; Siwicki et al., 1994), enhance phagocytic activity in the gilthead sea bream (Esteban et al., 2000, 2001; Ortuno et al., 2000; Cuesta et al., 2003), immersion and dietary supplements (Kono et al., 1987; Kawakami et al., 1998) and burn wound healing in rats (Alemdaro?lu et al., 2006).
However, the antioxidant effects of chitosan in flounder fish have not been investigated. Oxidative stress is believed to be the major biological process that mediates many diseases. Reactive oxygen species (ROS) can damage most biological molecules, including proteins, lipids and cellular DNA (Dalle-Donne et al., 2003). As a consequence, a non-specific defense system is required for all vertebrates in the aquaculture industry. Physical barriers, and protein and cellular defenses are used for protection against environmental stresses. Therefore, the main objective of this study was to determine the growth performance and the effects of a physical barrier, chemical defenses, and cellular factors including the cytoprotective efficacy of the chitosan-coated MP diet against oxidative stress in the olive flounder. We report the effects on lysozyme activity, mucus protein content, protein oxidation and oxidative damage of a chitosan-coated MP diet in olive flounder.
>
Experimental design and fish specimens
Olive flounder
>
Experimental diets and feeding
The fish were fed commercial MPs treated with chitosan-coating solution (20 g/kg chitosan, 10 g/kg tamarind gum, 5 g/kg vitamin C, 3 g/kg glucosamine sulfate, 1 g/kg chito-oligosaccharide, and 5 g/kg astaxanthin) which was sprayed onto the surface of the MP at a flow rate of 1,000 mL/min. Chitosan and chito-oligosaccharides were purchased from Kittolife Co., Ltd (Pyongtaek, Korea). The coated MPs were stored for 11-24 h at ?20℃. In the first feeding experiment, fish were divided randomly into two groups: 1) a control (non-coated MP diet) group and 2) a group fed a chitosan-coated MP diet. In each group (350 fish), the first feeding experiment was performed in triplicate individual tanks. Sanitation was manipulated by removing ~10% of the total water daily, along with the waste feed and fecal material. Feed was provided by hand twice per day (07:00 and 17:00), 7 days per week, for 12 weeks, to apparent visual satiety at a rate of 7% of the body weight. Uneaten feed was gently removed by siphoning and subtracted from the amount provided for the calculation of FCR and FI.
>
Proximate chemical composition of olive flounder
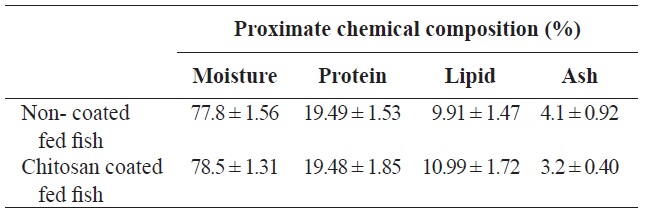
The proximate chemical composition of fish was determined according to standard Association of Official Analytical Chemists (AOAC) methods (Association of Official Analytical Chemists, 1995) after the 12-week experimental periods. Crude protein content was determined by the Kjeldahl method (Kejltec 8400; FOSS, Eden Prarie, MN, USA). Crude lipid analysis was performed by soxhlet extraction with diethyl ether solvent (Soxtec 2050; FOSS), and crude ash content was determined by incineration of samples at 550℃ in a muffle furnace (B180; Nabertherm GmbH, Lilienthal, Germany). The moisture content was determined by an oven-drying method at 105℃ using a moisture analyzer (mb45; Ohaus, Nanikon, Switzerland).
Blood was obtained from six individual fish, randomly selected from each replicated group at the end of the 12-week feeding trial after the overnight fasting period; bioassays were then performed. The fish were anaesthetized (3-aminobenzoic acid ethyl ester, MS 222; Sigma-Aldrich, St. Louis, MO, USA) and blood was collected from the caudal vein using a 22-gauge needle and a 3-mL syringe. The blood was then divided into two aliquots. One was heparinized, and the other was permitted to clot for 30 min at room temperature. Two blood aliquots were stored at 4℃ for 3 h. The clotted samples were centrifuged for 10 min at 3,000 rpm at 4℃ to separate serum. The heparinized blood was immediately utilized for the comet and carbonyl assays.
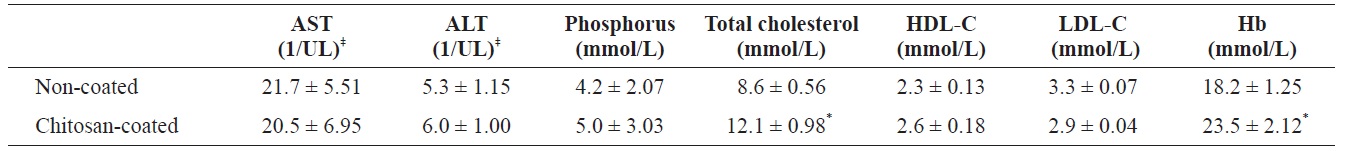
Biochemical assays were conducted using a blood chemistry auto-analyzer (ch100plus; Seac, Calenzano, Italy). The following biochemical parameters were assayed: aspartate aminotransferase (AST), alanine aminotransferase (ALT), phosphorus, total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL) and hemoglobin (Hb), using biochemical analysis kits (Stanbio Laboratory, Boerne, TX, USA).
>
Determination of lysozyme activity and total mucus protein content
The total skin mucus protein content was determined using a BCA protein assay kit (Pierce, Rockford, IL, USA) in accordance with the manufacturer’s protocols. The method described by Fagan et al. (2003), with some modifications, was used to collect skin mucus. Skin mucus was isolated using a scraper and suspended in five volumes of phosphate buffer saline (PBS; Gibco, New York, NY, USA). The samples were homogenized and centrifuged for 20 min at 12,000 rpm at 4℃. The serum samples were collected via exsanguinations from the caudal vein. Immediately after collection mucus and serum were stored at ?70℃ until use. A turbidometric assay was performed to determine lysozyme activity, employing lyophilized
Two hundred microliters of fresh whole blood were added to 1 mL of PBS (Gibco) and layered onto 200 μL of Histopaque 1077 (Sigma-Aldrich). After centrifugation for 30 min at 400
>
Comet assay (single-cell gel electrophoresis)
The alkaline comet assay was conducted according to the method of Heo et al. (2005). The cell suspension was mixed with 75 μL of 0.5% low melting agarose (LMA), and added to slides pre-coated with 1% normal melting agarose. After solidification of the agarose, the slides were covered with another 100 μL of 0.5% LMA, and then immersed in lysis solution (2.5 M NaCl, 100 mM ethylenediaminetetraacetic acid, 10 mM Tris, and 1% sodium lauroylsarcosine; 1% Triton X-100 and 10% dimethyl sulfoxide) for 30 min at 4℃. The slides were then placed into an electrophoresis tank containing 300 mM NaOH and 10 mM Na2EDTA (pH 13.0) for 20 min for DNA unwinding. For electrophoresis of the DNA, an electric current of 25 V/300 mA was applied for 20 min at 4℃. The slides were washed three times with a neutralizing buffer (0.4 M Tris, pH 7.5) for 5 min at 4℃, and then treated with 70% ethanol for a further 5 min before staining with 50 μL of ethidium bromide (20 μg/mL). Measurements were made by image analysis (Komet 5.0; Kinetic Imaging, Liverpool, UK) and fluorescence microscopy (BX-FLA; Olympus Optical Co. Ltd., Tokyo, Japan) was used to determine the percentage of fluorescence in the tail (tail intensity; 50 cells from each of two replicate slides)
>
Plasma protein carbonyl contents
Protein oxidation measured as an increase in dinitrophenylhydrazine (DNP)-reactive carbonyl groups, has been shown to be an early event in oxidative stress (Pacifici and Davies, 1990). In the study, protein carbonyls were estimated using 2, 4-dinitrophenylhydrazine (DNPH) based on an established procedure (Fagan et al., 1999). One hundred microliters of plasma mixed with 400 μL buffer and 500 μL of 10 mM DNPH (in 2 M HCl) were added and vortexed at 5 min intervals for 15 min at room temperature. Protein blanks were prepared by adding 500 μL of 2 M HCl instead of DNPH to the assay tubes. After mixing, 500 μL of 30% trichloacetic acid (TCA) were added to each tube, followed by vortexing and incubating on ice for 10 min. Following centrifugation (20 min at 800 g), the supernatant was discarded and the pellets were subjected to extensive washing with 5 mL of 20% TCA. The pellets were then washed with 5 mL of ethanol-ethylacetate (1:1, v/v) to remove unreacted DNPH and solubilized in 1 mL of 6 M guanidine hydrochloride and 20 mM potassium di-hydrogen phosphate (pH 2.3). The absorbance at 380 nm was recorded using a microplate reader (Spectracount Packard; Perkin Elmer Life and Analytical Sciences, Boston, MA, USA).
To detect oxidative DNA damage due to 8-hydroxyguanine, an ELISA Kit was used (JalCA Ltd., Shizuoka, Japan). Briefly, 50 μL of lymphocytes or a standard distributed with 50 μL of primary antibody per well was incubated at 37℃ for 1 h. Washing solution (250 μL) was then added to each well. The secondary antibody (100 μL) was then added, followed by incubation at 37℃ for 1 h. Thereafter, 100 μL of freshly prepared substrate solution were added, followed by incubation at room temperature for 15 min in the dark. Finally, the terminating solution (100 μL) was added and the absorbance at 450 nm was read using a microtiter plate reader (Spectracount Packard; Perkin Elmer Life and Analytical Sciences).
All assays were conducted in triplicate and the means ± SD
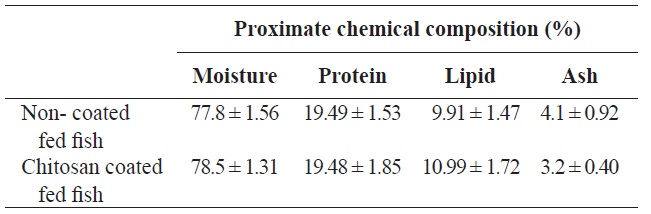
Proximate chemical composition (% on fresh-weight basis) of olive flounder Paralichthys olivaceus fed with chitosan-coated and non-coated diet for 12 weeks†
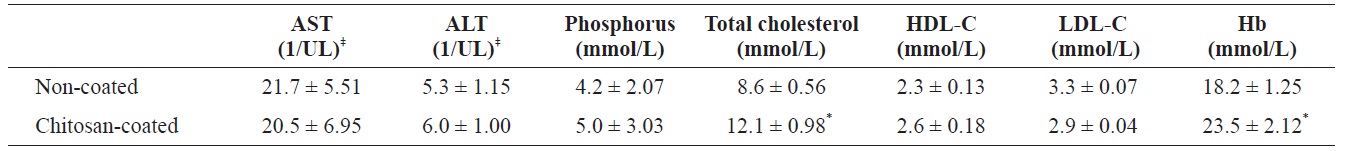
Serum analysis of olive flounder Paralichthys olivaceus that was fed with chitosan-coated and control (non-coated) diet after 12 weeks†
were recorded. Student’s
>
Proximate chemical composition and serum analysis of olive flounder
Following analysis, the moisture, protein, lipid and ash contents of the olive flounder were not significantly different between fish fed the two diets (chitosan-coated and non-coated MP) during the 12-week experimental period (Table 1). Serum analysis revealed that the total cholesterol and Hb levels of the fish fed the chitosan-coated diet were significantly higher than those of the control group. However, the mean low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), ALT, AST, and phosphorus levels were not significantly different compared to the control group (Table 2).
>
Growth performances and survival rate
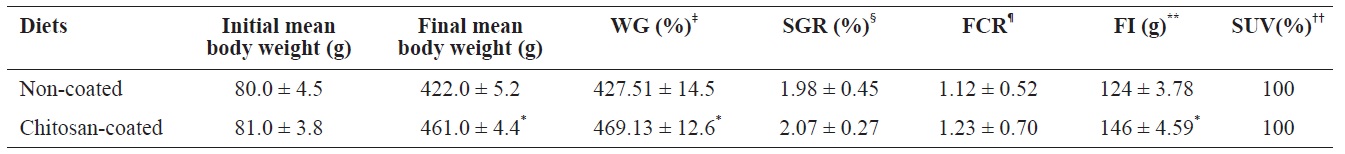
The growth performance and survival rate of olive flounder fish fed the chitosan-coated and non-coated MP diets for 12 weeks are presented in Table 3. During the experimental period, no mortality was reported in both fish groups; thus survivability was 100% in both groups. Olive flounder growth was evaluated over a 12-week experimental period at 2-week intervals. Fig. 1 shows the average weight gain (g/fish) of fish during the 12-week study period. The average weight gain was significantly greater in fish fed the chitosan-coated MP diet compared to the controls. Moreover, the final mean body weight (g) of the fish fed a chitosan-coated MP diet was significantly higher (461 ± 4.4 g) than that of the controls (422 ± 5.2 g) (Table 3). In addition, weight gain (%) and FI (g) increased significantly during the study period. Furthermore, SGR (%) and FCR values were higher in fish fed the experimental diet, albeit not significantly so.
>
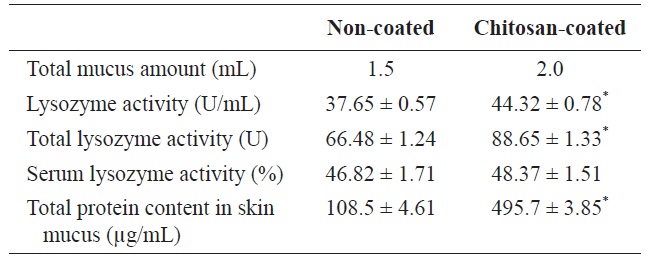
Lysozyme activity and total mucus protein content
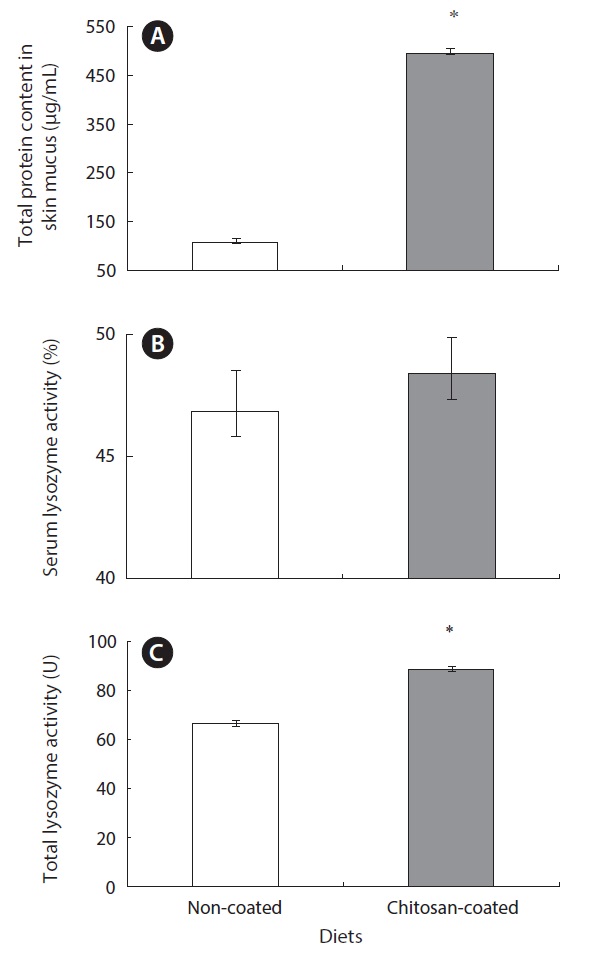
Lysozyme is an important hydrolytic enzyme in the nonspecific defense system. Fish skin mucus serves as a mechanical barrier and contains many substances with protective effects. Mucus lysozyme activity differed significantly between fish fed the experimental and control diets (Table 4). However, serum lysozyme activity did not differ significantly between the groups. However, serum lysozyme activity was higher in the fish fed the chitosan-coated MP diet compared to the control group. Interestingly, the total protein content of fish skin mucus increased markedly in the chitosan-coated MP group compared to the control group (Fig. 2). Therefore, a mechanical barrier is involved in olive flounder immunity in terms of protection against invading pathogens.
>
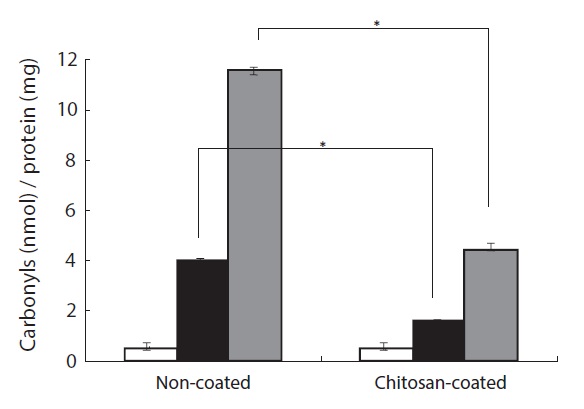
Plasma protein carbonyl contents
The protective effect of a chitosan-coated MP diet on H2O2- induced protein oxidative damage in the plasma of the olive flounder was investigated. Protein carbonyl formation serves as a biomarker of cellular oxidative stress damage (Stadtman, 1995). As shown in Fig. 3, protein carbonyl formation in plasma increased after H2O2 treatment in fish fed the chitosancoated and non-coated MP diets. In the group fed the chitosancoated MP the increase was approximately threefold greater than in the control group at 50 μM H2O2 and 12-fold at 100 μM. Furthermore, the protein carbonyl content after 50 μM
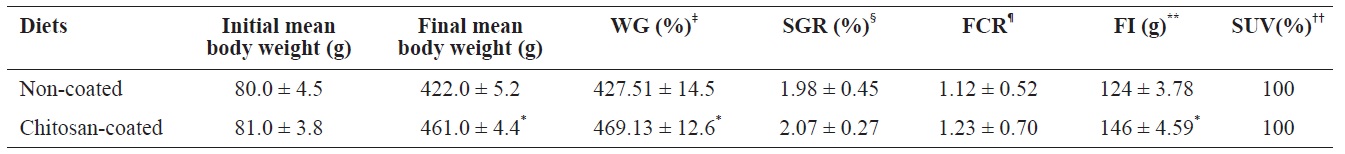
Growth performances of olive flounder (Paralichthys olivaceus) fed with chitosan-coated and non-coated diet for 12 weeks†
H2O2 treatment was twofold higher in fish fed the chitosancoated MP diet than in the control group, and approximately fourfold higher after te 100 μM H2O2 treatment. The group fed the chitosan-coated MP diet had a protein carbonyl content approximately three times lower than that of the group fed the non-coated MP diet at 100 μM H2O2 (Fig. 3). These results indicate that the chitosan-coated MP diet attenuated the H2O2- induced formation of protein carbonyl in the olive flounder.
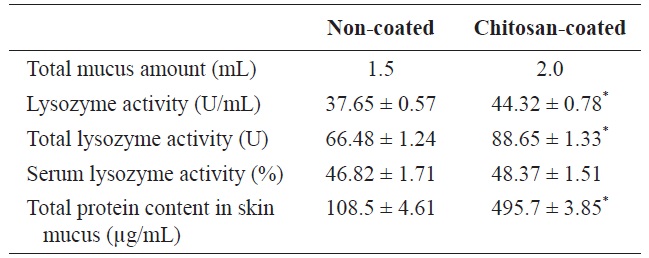
Immunological parameters of olive flounder (Paralichthys olivaceus) fed with chitosan-coated and non-coated diet for 12 weeks†
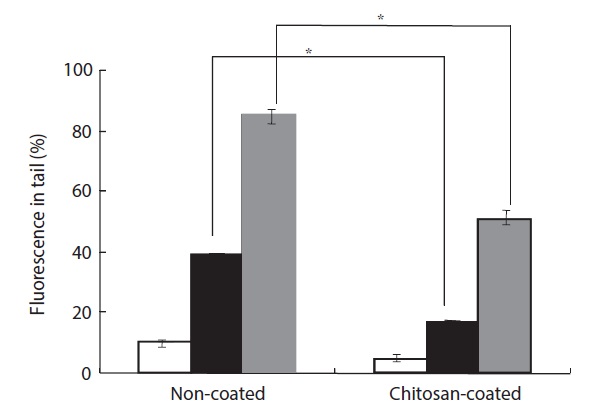
H2O2-induced cellular DNA damage was detected by both an alkaline comet assay and 8-OHdG formation. H2O2 plays a significant role because it is generated by all sources of oxidative stress and can diffuse freely in and out of cells and tissues. Moreover, cellular DNA is sensitive to the action of H2O2, and DNA damage can occur due to transition metal ions, particularly iron and copper (Barbouti et al., 2002). H2O2-induced cells were found to exhibit increased DNA damage in the comet assay. The percentage of DNA in the tail increased to 40% after treatment with 50 μM H2O2 compared to the group fed a non-coated MP diet (Fig. 4). However, in the group fed a chitosan-coated MP diet, the percentage of DNA in the tail decreased by 20%. In the group fed the non-coated MP diet, treatment with 100 μM H2O2 resulted in an 86% increase in DNA in the tail, while in the group fed the chitosan-coated MP diet the percentage of DNA in the tail decreased by 52%.
The DNA migration profiles are shown in Fig. 5. The length and intensity of the ‘comet tail’ corresponds to the amount of DNA damage. The amount of tail DNA increased substantially with an increasing concentration of H2O2 in both groups (Fig. 5C, 5D and 5E, 5F), compared to the control (Fig. 5A and 5B). However, the amount of tail DNA was lower in fish fed the chitosan-coated MP diet group than in those fed the non-coated MP diet (Fig. 5C, 5E and 5D, 5F).
8-OHdG is one of the major products of ROS-induced DNA damage. It can be used as a biomarker of oxidative DNA damage following the increase of H2O2-treated lymphocytes in the olive flounder. As shown in Fig. 6, the cells of fish fed the non-coated MP diet exhibited a 2.9-fold greater 8-OHdG content following 100 μM H2O2 treatment compared to the control. In addition, the 8-OHdG content of of fish fed the chitosan-coated MP diet was increased by 1.8-fold compared to the control following 100 μM H2O2 treatment. Moreover, the fish fed the chitosan-coated MP diet displayed twofold lower 8-OHdG formation than the fish fed the non-coated MP diet following 100 μM H2O2-treatment. Taken together, these results suggest that feeding the chitosan-coated MP diet attenuated H2O2-induced cellular DNA damage.
Our results indicate that olive flounder growth performance, feed utilization and physiological status were markedly affected by the chitosan-coated MP diet. Growth performance and feed utilization vary according to fish species, environmental conditions, available nutrients, and stage of development (Silva et al., 2007). In this study, the weight gain (%) was 469.13 ± 12.6% in the experimental group (final mean body weight of fish fed a chitosan-coated MP diet; 461 g), which differed significantly compared to the control 427.51 ± 14.5% (final mean body weight of control fish; 422 g) under identical environmental conditions. This can be attributed to the FI and the effects of nutrients in the experimental diet during the 12-week period. The survival rate of fish increased markedly, and no mortality occurred in either group.
Measurements of physiological parameters are commonly used as diagnostic tools in aquatic toxicology and bio-monitoring systems (McDonald and Milligan, 1992; Folmar et al., 1993; Kang et al., 1995; Soimasuo et al., 1995; Harikrishnan
activet al., 2003). Serum biochemical parameters have provided evidence of positive health effects in fish fed a chitosancoated MP diet. In particularly, Hb, which carries oxygen into the organs, and total cholesterol levels increased markedly and differed significantly compared to the control. However, levels of HDL (unsaturated fatty acid) were higher and levels of LDL (saturated fatty acid) were lower compared to the control, albeit not significantly so. AST (an indicator of liver toxicity) levels were also lower in fish fed the chitosan-coated MP diet. Kim et al. (2012) reported that major hematological parameters decreased significantly following two intraperitoneal injections of mercuric chloride (2, 4, or 8 mg Hg/kg BW). Thus positive effects were noted throughout the experimental period, and the general health of fish improved.
The analysis of the proximate chemical composition of the olive flounder fish revealed no significant differences between the experimental and control groups. However, due to the food intake, high nutrient utilization, nutrient digestibility, and nutrient deposition were also linked to the growth performance and high survivability of fish throughout the 12-week study period.
Interestingly, the immunological parameters measured indicated pronounced effects on serum and mucus lysozyme activity in fish fed the chitosan-coated MP diet. In the innate immune system of the fish, a physical barrier acts as the first line of defense against infection and inflammation. Fish skin mucus may play an important role in this defense. In this study, the total protein content of fish skin mucus was significantly higher (495.7 ± 3.85 μg/mL) in fish fed the chitosan-coated MP diet compared to those fed the non-coated MP diet (108.5 ± 4.61 μg/mL). This indicates the protective effect of mucus components, including lectins, lysozyme, complement proteins, antibacterial peptides, and IgM (Rombout et al., 1993; Aranishi and Nakane, 1997). Importantly, this provides insight into the constituents of the mucus proteins that protect against pathogenic bacteria and viruses. Lysozymes attack the cell wall components, such as peptidoglycans, of gram-positive bacteria and break the connection between N-acetylmuramic acid and acetylglucosamine, leading to lysis of the bacteria. A turbidometric assay was used to determine the lysozyme activity of serum and mucus of fish fed both a chitosan-coated and non-coated MP diet during the 12-week study period. To assay lysozyme activity, the cell wall of
Reactive oxygen species, such as hydrogen peroxide (H2O2), superoxide anions (O2?), singlet oxygen (1O2), and hydroxyl radicals (OH?), are unstable reactive molecules that are involved in various pathologies (Sundaram and Panneerselvam, 2006). H2O2 is one of the major ROS related to oxidative stress. It facilely penetrates cells and reacts with intracellular ions such as iron and copper. This leads to generation of highly reactive hydroxyl radicals, which damage lipids, proteins, and DNA (Aruoma, 1998). However, H2O2 is considered to be an environmentally sustainable material, because it decomposes readily into water and gaseous oxygen. Hence, it is classified as a ‘low regulatory compound’ by the Food and Drug Administration (FDA) of the United States (Avendano-Herrera et al., 2006). Therefore, identifying alternative natural substances that protect against ROS for use as a feed material in the aquaculture industry is of interest.
The fragmentation of polypeptide chains, formation of protein-protein cross linkages, and modification of amino acid side chains to hydroxyl or carbonyl derivatives are all possible outcomes of oxidative reactions. Therefore, the carbonyl content of proteins is an indicator of oxidative protein damage. Measurement of protein oxidation offers several advantages over monitoring of lipid peroxidation, such as the early formation and relative stability of oxidized protein (Pantke et al., 1999). Oxidative damage to proteins may be a more critical pathological event than damage to lipids because proteins are possible intermediate vehicles and catalysts in the biological reactions that mediate oxidative stress in the cells (Dean et al., 1997). In addition, protein carbonyl groups react with DNPH to form a stable derivative as a DNP hydrazone complex, which can be detected by a colorimetric method (Dalle-Donne et al., 2003).
In this study, carbonyl formation in the plasma of olive flounder fed a chitosan-coated MP diet was significantly lower than in the control group after H2O2 treatment. In particular, the carbonyl level in the plasma of fish fed a chitosan-coated MP diet was threefold lower than that in control fish after 100 μM H2O2 treatment. Thus the chitosan-coated MP diet prevented H2O2-induced protein carbonyl formation. Blood plasma is easily obtainable from animals and humans and contains both lipid and protein components that may be susceptible to oxidation. Hence, a physicochemical environment appropriate for investigation of plasma oxidation variables using biomarkers can be used to identify
DNA damage is a sensitive biological marker of oxidative stress, and represents the imbalance between free radical generation and the activity of the antioxidant systems (Park et al., 2005). Moreover, DNA is damaged by oxidative stress, which may result in modification of bases or the breaking of the DNA strands. In addition, deoxyguanosine (dG) is modified to 8-hydroxyguanine (oh8Gua) by incorporation of a hydroxyl group at position eight. This reaction occurs readily in the presence of oxidative stress, and oh8Gua can then be modified to 8-oxoguanine by addition of a ketone group (Kasai and Nishimura, 1984). Oh8Gua is generally used as a marker of oxidative stress (Kasai, 1997), and causes mainly GC to TA transversions, leading to mutagenesis or carcinogenesis in mammalian cells (Grollman and Moriya, 1993).
H2O2-induced DNA damage was also detected by means of an alkaline comet assay and 8-OHdG formation. The percentage of DNA in the tail was significantly lower in fish fed a chitosan-coated MP diet than in the control group (Figs. 4 and 5). The tail DNA of fish fed the non-coated MP diet was destroyed after 100 μM H2O2 treatment. However, the amount of tail DNA was lower in fish fed a chitosan-coated MP diet (Fig. 5). Cells exposed to H2O2 had increased 8-OHdG contents (Fig. 6). In addition, the fish fed a chitosan-coated MP diet exhibited lower 8-OHdG formation than the control fish. The antioxidative activity of chitosan formulations has been investigated extensively. The strong hydrogen-donating ability of chitosan has led to its use as an antioxidant. In addition, the chitosan nano-particles exhibit a tremendous loading capacity and biocompatibility, and there is evidence that they bind to target sites (Jarmila and Vavrikova, 2011).
The chitosan formulation comprises monosaccharide subunits and N-acetyl-D-glucosamine residues, which facilitate binding to mucosal or serum agglutinins and precipitins, which are C-type lectines and pentraxins. The interactions of these molecules may lead to activation of the complement system (Magnadottir, 2006). Our findings also indicate that the chitosan-coated MP diet effectively induced innate immune defenses in olive flounder. In addition, the positive effects on the blood parameters and the protective immunological and physiological effects demonstrate that the chitosan-coated MP diet may be used to manipulate the growth performance and survivability of olive flounder. Taken together, these results suggest that feeding of a chitosan-coated MP diet could be a practical method for aquaculture systems to enhance the innate immune system of the olive flounder. Such a diet will also ensure a satisfactory health condition and growth performance by protecting the olive flounder from environmental stresses during aquaculture.