Among cancer, gastric cancer causes nearly one million deaths worldwide per year. Gastric cancer is one of the leading causes of cancer-related death worldwide due to its frequency, poor prognosis and limited treatment options. Although the incidence of gastric cancer has been declining for several decades in most countries, it remains a crucial public health problem in some countries [1].
In previous studies, we suggested that human gastric adenocarcinoma cells expressed the transient receptor potential melastatin 7 (TRPM7) channel, which is essential for cell survival and is a potential target for pharmacological gastric cancer treatment [2]. TRPM7 is endogenously expressed in a wide variety of tissues including brain and hematopoietic tissues [3], as well as kidney and heart tissues [4-6]. The TRPM7 cation channel supports multiple cellular and physiological functions, including cellular Mg2+ homeostasis [7, 8], cell viability and growth [811], anoxic neuronal cell death [12], synaptic transmission [13], cell adhesion [14, 15], and intestinal pacemaking [16]. Wykes
The BMKNE used in this study were purchased from the Korea Research Institute of Bioscience and Biotechnology (KRIBB).
AGS lines were used. The AGS cell lines were established at the Cancer Research Center, College of Medicine, Seoul National University, Korea. The cell lines were propagated in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco-BRL) supplemented with 10% heat-inactivated fetal bovine serum and 20-μg/mL penicillin and streptomycin in an atmosphere of 5% CO2 at 37℃.
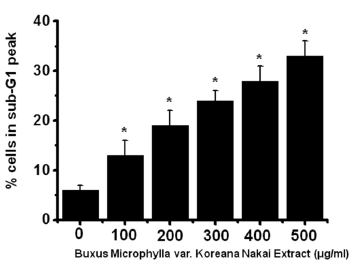
In order to investigate whether the cell cycle of AGS cells was redistributed, we used a flow cytometric analysis with propidium iodine (PI) stain [21, 22]. We placed 1 × 106 cells in an e-tube. An ice-cold fixation buffer (ethyl alcohol), 700 μL was slowly added with vortexing. The tubes were sealed with parafilm and incubated at 4℃ overnight. Samples were spun for 3 min at 106 g at 4℃, and the supernatant was aspirated and discarded. The cell pellet was resuspended in 200 μL of PI staining solution (PI [5 mg/mL] 2 μL and RNase 2 μL in PBS 196 μL) at 20817 g for 5 s. After 30 min in the dark at room temperature, the samples were analyzed in a fluorescence activated cell sorter (FACScan; Becton-Dickinson, Moutain View, CA, USA) at λ= 488 nm by using Cell-Quest software (Becton-Dickinson). The DNA content distribution of normal growing cells was characterized by using two peaks, the G1/G0 and the G2/M phases. The G1/G0 phase comprises the normal functioning and resting state of the cell cycle with the most diploid DNA content, while the DNA content in the G2/M phase is more than diploid. Cells in the sub-G1 phase have the least DNA content in the cell cycle distribution; this is termed hypodiploid. Hypoploid DNA contents represent the DNA fragmentation [23].
2.4. MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assay
Cell viability was assessed by using a MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide) assay. The AGS cells were seeded into each well of 12-well culture plates and then cultured in Roswell Park Memorial Institute medium (RPMI)-1640 supplemented with other reagents for 72 h. After incubation, 100 μL of MTT solution (5 mg/mL in phosphate-buffered saline (PBS)) was added to each well, and the plates were incubated at 37℃ for 4 h. After the supernatant had been removed and shaken with 200 μL of dimethyl sulfoxide (Jersey Lab Supply, Livingston, NJ, USA) for 30 min, the absorbance was measured at 570 nm. All experiments were repeated at least 3 times.
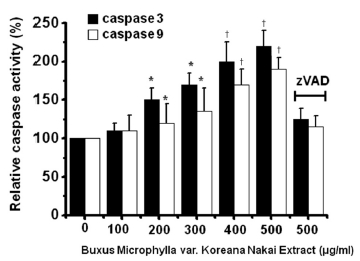
Caspase-3 and -9 assay kits (Cellular Activity Assay Kit Plus) were purchased from BioMol (Plymouth, PA, USA). After experimental treatment, cells were centrifuged (10000 g, 4℃, 10 min) and washed with PBS. Cells were re-suspended in ice-cold cell lysis buffer and incubated on ice for 10 min. Sample were centrifuged at 10000 g (4℃, 10 min), and the supernatant was removed. Supernatant samples (10 μL) were incubated with 50 μL of substrate (400-μM Ac-DEVD-pNA) in 40 μL of assay buffer at 37℃. The absorbance at 405 nm was read at several time points. The pNA concentrations in the samples were extrapolated from a standard created using the absorbances of sequential pNA concentrations.
2.6. Assessment of mitochondrial membrane depolarization
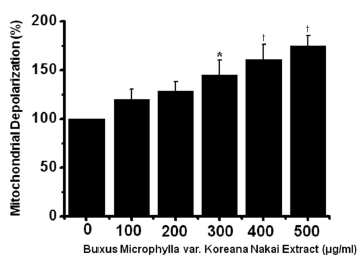
Mitochondrial membrane depolarization was evaluated using a JC-1 fluorescence probe according to the manufacturer’s instructions (Santa Cruz). The AGS cells were labeled with 2 μM JC-1 for 30 min at 37℃ and then analyzed by using flow cytometry with 488-nm excitation and 530/30- or 585/42-nm bypass emission filters. The cells without red fluorescence were regarded as the cells manifesting mitochondrial membrane depolarization.
A whole-cell configuration of the patch-clamp technique experiment was performed at room temperature (22?25℃). The AGS cells were transferred to a small chamber on an inverted microscope stage (IX70, Olympus, Japan) and were constantly perfused with a solution containing 2.8-mmol/L KCl, 145-mmol/L NaCl, 2-mmol/L CaCl2, 10-mmol/L glucose, 1.2-mmol/L MgCl2, and 10-mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), adjusted to a pH of 7.4 with NaOH. The pipette solution contained 145-mmol/L Cs-glutamate, 8-mmol/L NaCl, 10-mmol/L Cs-2-bis (2-aminophenoxy)- ethane-N,N,N′,N′-tetraacetic acid, and 10-mmol/L HEPES-CsOH, adjusted to a pH of 7.2 with CsOH. Axopatch I-D (Axon Instruments, Foster City, CA, USA) was used to amplify the membrane currents and potentials. For data acquisition and the application of command pulses, pCLAMP software v.9.2 and a Digidata 1322A units (Axon Instruments) were used. Results were analyzed using pClamp and Origin software (Microcal Origin version 6.0).
2.8. TRPM7 expression in human embryonic kidney-293 cells
Human embryonic kidney (HEK)-293 cells were transfected with the Flag-murine long transient receptor potential channel 7 (LTRPC7)/pCDNA4-TO construct and grown on glass coverslips in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, blasticidin (5 μg/mL), and zeocin (0.4 mg/mL). TRPM7 (LTRPC7) expression was induced by adding 1 μg/mL of tetracycline to the culture medium. Whole-cell patch-clamp experiments were performed at 21-25℃ with cells that had been grown on the glass coverslips.
Data are expressed as mean ± standard error of the mean (SEM). Differences between the data were evaluated by using the student’s
3.1. BMKNE-induced cell death in AGS cells
To ascertain whether BMKNE kills AGS cells, we performed MTT assays. The viable cell population was gradually reduced with increasing concentrations of BMKNE for 24 h in AGS cells (Fig 1) Thus, our results demonstrate that BMKNE induces cell death in AGS cells.
3.2. BMKNE-triggered apoptosis in AGS cells
To determine whether AGS cell death occurs by apoptosis, we conducted sub-G1 analysis [24, 25]. In this protocol, cells were incubated with BMKNE and stained with a fluorescent DNA stain (PI). The action of endogenous endonucleases in apoptotic cells cleaves DNA into endonucleosomal fragments of typical size, which are extracted from the cells. The loss of DNA is detected by using an FACS analysis, as the reduced nuclear staining in apoptotic cells, which results in a novel (sub-G1) fluorescence peak to the left of the regular fluorescence peak. Flow cytometric analysis showed that the percentage of sub-G1 phase cells markedly increased in the AGS cells treated with BMKNE in a dose-dependent manner (Fig 2) In addition, BMKNE elevated mitochondrial membrane depolarization, an early event of an intrinsic apoptosis signaling (Fig 3) Thus, our findings suggest that BMKNE induces apoptosis via intrinsic apoptotic mechanism(s). Caspase-3 and -9 activations are hallmarks of apoptotic cell death. We also measured the enzyme activity in AGS cells after BMKNE incubation. Using a synthetic substrate, we detected the caspase-3 and -9 activities in AGS cells. BMKNE increased the activities of caspase-3 and -9 (Fig 4)
To investigate the signaling pathway of BMKNE-induced apoptosis in AGS cells, we assessed the effect of BMKNE on mitogen-activated protein kinases (MAPKs), because they play critical roles in the apoptosis-related signaling pathway. As shown in (Fig 5), exposure to BMKNE with c-jun NH2-terminal kinase (JNK) II inhibitor (Fig 5)A) or SB203580 (a p38 MAPK inhibitor) (Fig 5)B) resulted in increases in the viable cell populations.
3.4. Effects of BMKNE in TRPM7 currents in AGS- and TRPM7- overexpressed HEK293 cells
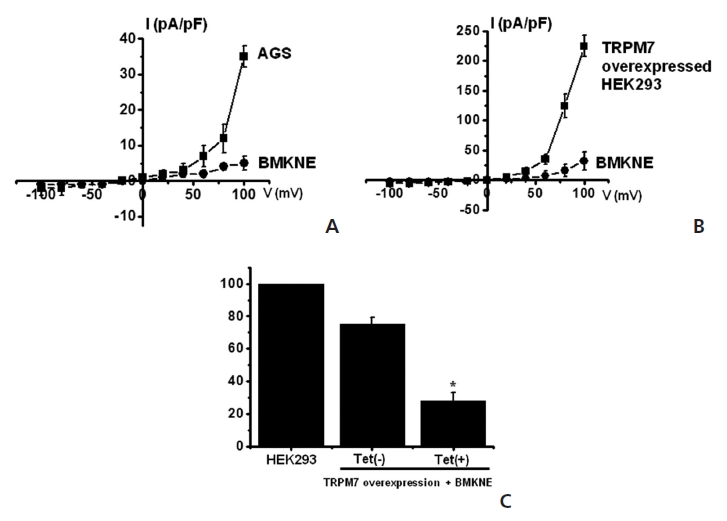
TRPM7 has been proposed to be required for cell survival on the basis of experiments on genetically-engineered DT-40 B-cells [9]. Also, we recently suggested, as in previous reports, that AGS cells expressed the TRPM7 channel and that suppression of the TRPM7 channel induced cell death [2]. Therefore, we investigated whether BMKNE influenced TRPM7 currents in AGS cells. To confirm the effect of BMKNE in TRPM7 currents, we investigated the effects of BMKNE in AGS cells by using patch-clamp techniques. We performed whole cell voltage-clamp recordings to investigate the effect of BMKNE on the TRPM7-like current in AGS cells. A voltage ramp of from +100 mV to -100 mV evoked small inward currents at negative potentials, whereas larger outward currents were evoked at positive potentials, showing that the currents were outward-rectifying cation currents (n = 4; (Fig 6)A). However, in the presence of 500 μg/ml of BMKNE, the amplitudes of these currents were inhibited outwardly by 92.3 ± 2.4% and inwardly by 95.1 ± 2.1% (n = 4; (Fig 6)A). Also similar results were obtained in HEK293 cells over-expressing TRPM7 (Fig 6)B). To provide additional evidence that supports the contribution of the TRPM7 channel to BMKNE toxicity, we investigated changing expression levels of the TRPM7 channel and its influences on BMKNE-mediated cell death. We used HEK293 cells with inducible TRPM7 channel expression [18]. In the absence of induced TRPM7 channel expression [TRPM7(-) cells, Tet(-)], HEK293 cells incubation with BMKNE induced cell death, as observed on the MTT assay (n = 4; (Fig 6)C). However, when TRPM7 channel overexpression was induced by adding tetracycline [TRPM7(+) cells, Tet(+)], HEK293 cells incubation with BMKNE induced cell death at an increased rate in the MTT assay, which suggests that increased expression of TRPM7 channels leads to an increased rate of BMKNE-induced cell death.
In this study, we demonstrated that BMKNE suppresses AGS cell growth in culture models. Molecular and cellular analyses showed that the anticancer activity of BMKNE is attributable to cell cycle arrest and/or apoptosis. In addition, we found that BMKNE-induced apoptosis was inhibited by MAPK inhibitors and was induced by the blockade of TRPM7 channels. Our results indicate that BMKNE is a useful agent for pharmacological approaches for future development of anticancer drugs [26]. In addition, our findings suggest that BMKNE would be a useful drug tool for approaches to identify novel therapeutic targets for gastric cancer.
Apoptotic cell death pathways are induced by a variety of signals. One mechanism that is consistently implicated in apoptosis is the activation of a series of cytosolic proteases, the caspases [30]. Caspases are synthesized as inactive proenzymes that are processed in cells undergoing apoptosis by self-proteolysis and/or cleavage by another protein [2]. BMKNE has been used as a folk remedy for several diseases, including malaria and venereal diseases. However, only a few reports have described the effects of BMKNE on gastric cancer.
In summary, BMKNE inhibited the growth and the survival of AGS cells. Sub-G1 and caspase-3 and -9 activities were elevated, and the mitochondrial membrane depolarization potentials were increased. MAPK signaling was involved in BMKNE-induced apoptosis. TRPM7 currents were inhibited by BMKNE. Furthermore, overexpression of TRPM7 channels in HEK 293 cells increased the rate of BMKNE-induced cell death. These findings indicate that BMKNE inhibits the growth and the survival of gastric cancer and that BMKNE-induced apoptosis is due to the blockade of the TRPM7 channel’s activity and is involved in MAPK signaling. Therefore, BMKNE may be a potential drug for the treatment of gastric cancer.
![Buxus Microphylla var. Koreana Nakai Extract (BMKNE) induces cell death in AGS cells. MTT (3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide)?based viability assay. The AGS cells were treated with increasing concentrations of BMKNE for 24 h. Distilled water was used as a vehicle. Cell viability is expressed as a value relative to that of the untreated cells which is set to 100%. The figures show means ± SEMs. *P < 0.01.](http://oak.go.kr/repository/journal/12429/DHOCBS_2013_v16n3_39_g001.jpg)