Entomopathogenic fungi are natural pathogens of insects and contribute to the regulation of host insect populations in the natural environment. Their mode of action against insects involves the attachment of conidia to the insect cuticle followed by germination, cuticle penetration, and internal dissemination throughout the insect (Vega
Recently, secondary metabolites isolated from entomopathogenic fungi have been reported as potential bioactive substances (Isaka
These findings confirm the potential of entomopathogenic fungi as sources of lead compounds in pharmaceutical interests. Therefore, in this study, we report our results by assessing the ability of entomopathogenic fungi isolated in Korea (Shin
>
Entomopathogenic fungal metabolites
Entomopathogenic fungi, which exhibit virulence against the great wax moth (
For quantitative assay, all fungal conidia were obtained by scraping a 2-week-old PDA plate and suspended in a 0.05% Tween-80 solution. The conidial suspension was vigorously agitated and filtered through cotton to remove mycelial debris. After counting the number of conidia, 50 μl of the conidial suspension (2 × 106 conidia/ml) was inoculated with 20 ml of SDYB medium in a 100-ml Erlenmeyer flask and cultured using the abovementioned method. After 10 days, the samples were centrifuged at 13,000 rpm for 20 min. The pellet was removed and the supernatant was filtered using a 0.45 μm membrane
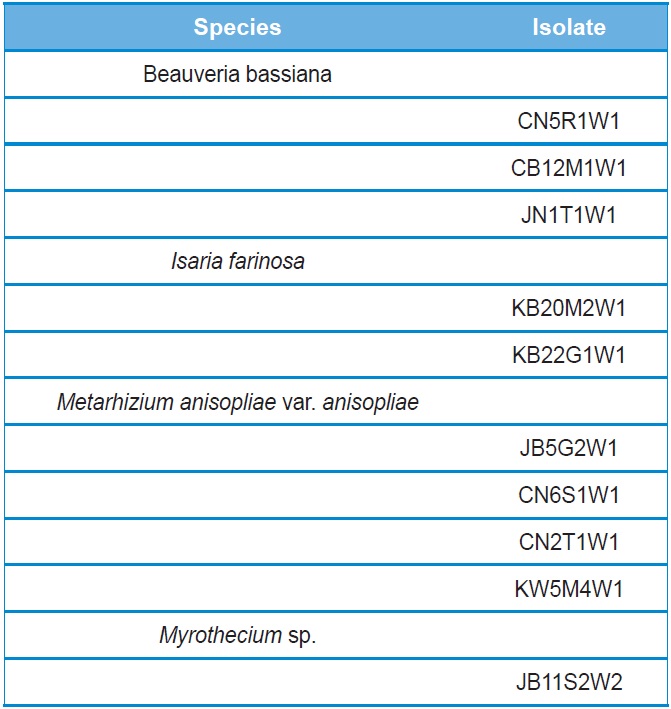
[Table 1.] Entomopathogenic fungal isolates used in this study
Entomopathogenic fungal isolates used in this study
filter (ADVACTEC No. 2) to separate the crude extract from the mycelium and spore mass. All fungal cell-free culture filtrates were stored at -70℃ until antibacterial activity could be detected.
For the preparation of bacteria,
>
Antibacterial activity assay
Antibacterial activity against
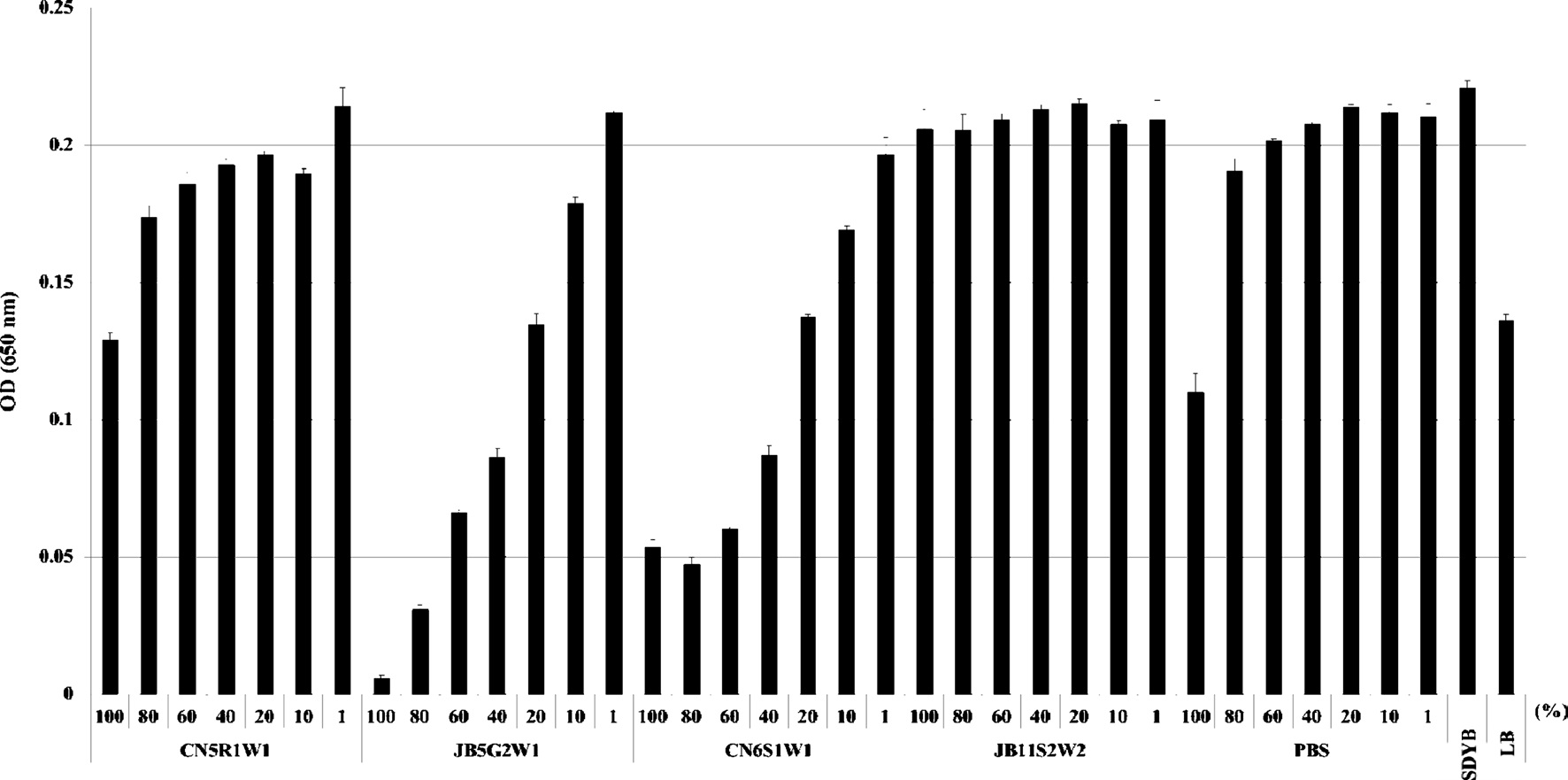
For the quantitative assay, 100 μl of bacterial suspensions were placed in 96 wells and then mixed with 100 μl of the fungal culture filtrate at different concentrations (i.e., 1%, 10%, 20%, 40%, 60%, 80%, and 100%). The fungal culture filtrate was diluted with SDYB media. For the control, bacterial suspensions were cultured in SDYB media with different concentrations of PBS in place of the fungal culture filtrate. All experiments were performed in triplicate.
Bacterial cell viability was assessed by MTT [3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay based on the reduction of MTT to formazan dye by active mitochondria. Briefly, 20 μl of the MTT solution (5 mg/ml in PBS) was added to each well and incubated for 4 h. Then, the MTT-spiked media was carefully removed by centrifugation at 13,000 rpm for 10 min, and formazan formed in the cells was dissolved in 200 μl of dimethyl sulfoxide. Absorbance at 540 nm was read using a micro plate reader (Molecular Devices, UK).
>
Stability of antibacterial activity of culture filtrate
For determining the heat stability, the culture filtrate was treated at various temperatures (50℃, 80℃, 100℃, and 121℃) for 15 min. After thermal shock, the culture filtrate was quickly cooled to 25℃, and its antibacterial activity was evaluated.
To test its stability in the presence of a protease, the culture filtrate was treated with proteinase K (Sigma, USA) at a final concentration of 1 mg/ml under the condition of 37℃ for 2 h. Then, the culture filtrate was autoclaved at 121℃ for 15 min to inactivate the enzymes before the antibacterial activity assay was conducted.
>
Screening of fungi showing antibacterial activity
Primary screening for the antibacterial activity of entomopathogenic
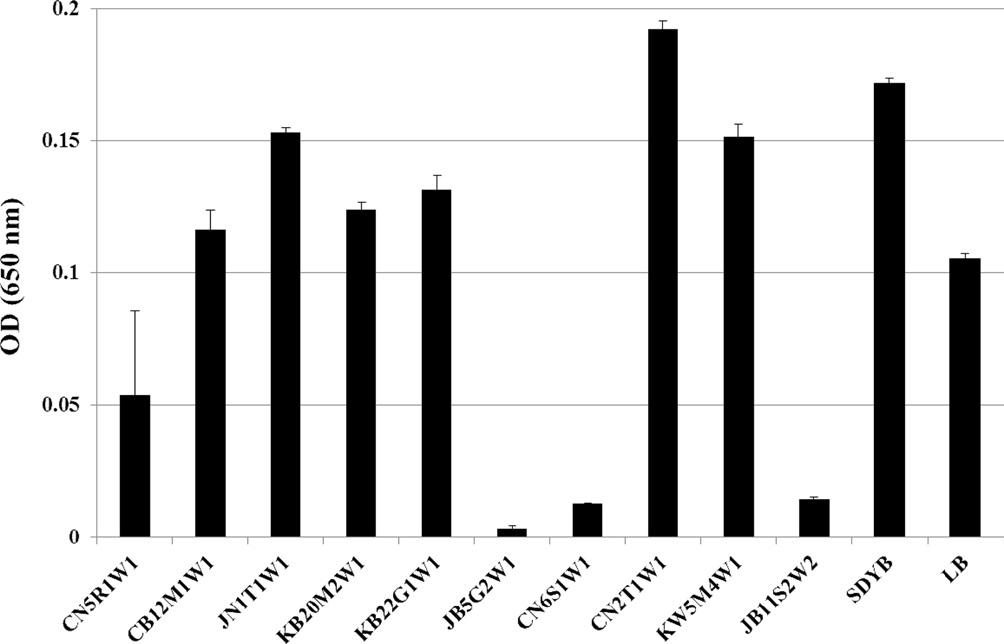
fungal isolates was carried out using a broth dilution assay. Among the 10 isolates tested, the culture filtrate of 4 isolates (i.e.,
>
Quantitative assay of antibacterial activity
Fungi were selected for scale-up culture with the same concentration of conidia and they were them subjected to a quantitative assay. The culture filtrate of
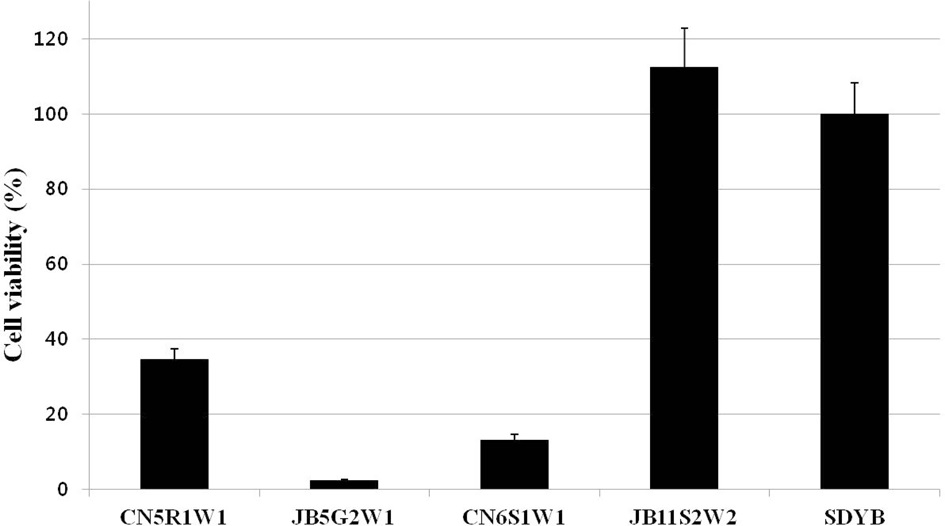
Surviving bacterial cell numbers in each well treated with 100% of the culture filtrate were determined indirectly by MTT
assay. As a result, for all fungal culture filtrates, no differences were detected between the O.D. (Fig. 2) and cell viability values (Fig. 3) obtained by both assays.
>
Stability of antibacterial activity
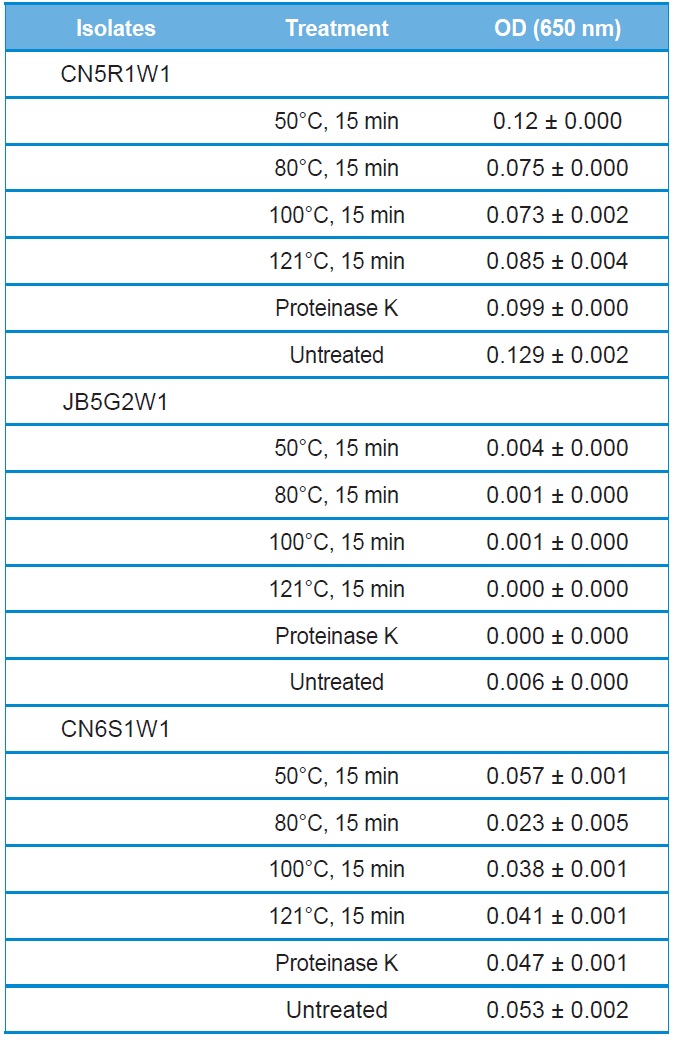
The stability of culture filtrates to heat and protease treatments were evaluated for antibacterial activity. Heat treatment did not influence the antibacterial activity of the culture filtrate for all tested isolates (Table 2). Furthermore, the culture filtrate retained its antibacterial activity even after treatment at 121℃ for 15 min. The hydrolytic enzyme, proteinase K, also had no effect on the antibacterial activity of all culture filtrates (Table 2).
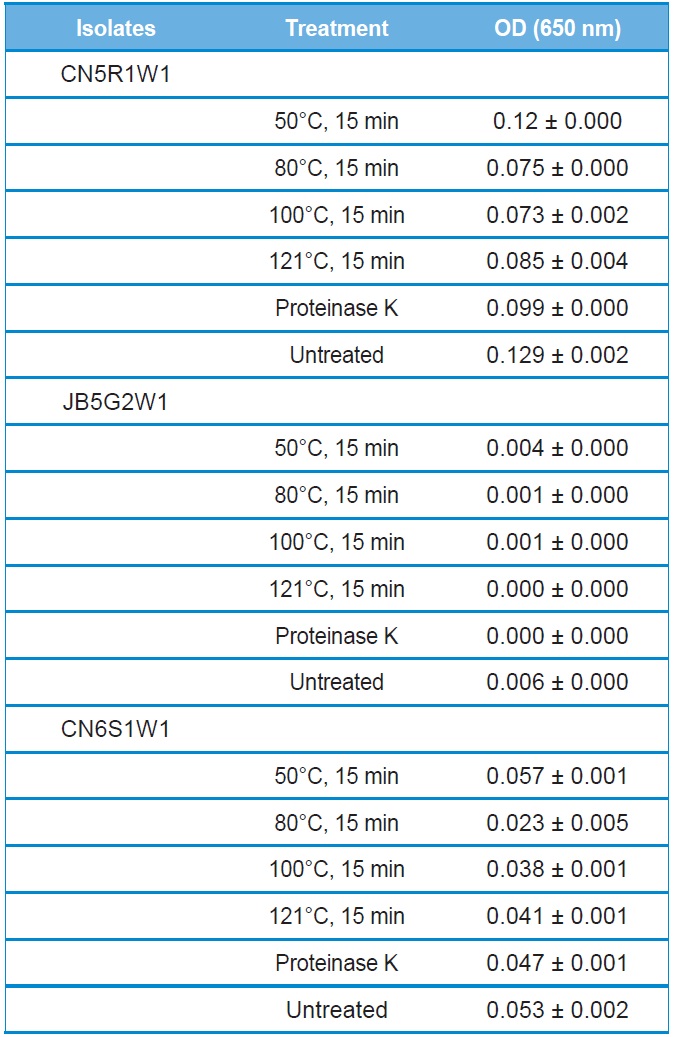
Effect of temperature and enzymatic shock on the antibacterial activity of culture filtrates of entomopathogenic fungi. Data represent mean values of 3 replicates with standard error
was not determined, but it was determined and inoculated in the quantitative assay. The conidial concentration for inoculation may have been insufficient to produce a substantial yield of antibacterial compounds from
To determine whether the antibacterial activity of the fungal culture filtrate showed cytotoxic or cytostatic effects against
Antibacterial compound(s) produced from selected fungal isolates remained stable in response to heat and proteolytic enzyme treatments (Table 2). These results suggest that the antibacterial compound(s) is not a protein. Heat-stable antimicrobial compounds are not commonly produced. Some Class II bacteriocins have been reported to be heat stable (Bharti
Fungi produce a wide range of compounds with biological activities against other organisms; most of these are products of secondary metabolites (Vey