Bees are both widely diverse and abundant, with 16,325 identified species worldwide (Michener, 2000). Bees are critical for the pollination of natural vegetation and agricultural plants, including fruits, vegetables, seed plants, edible oil crops, garden flowers, and major forage crops. Pollination is therefore an ecosystem service insofar as wild pollinators, particularly wild bees, contribute significantly to the pollination of a wide range of crops (Morandin and Winston, 2005; Greenleaf and Kremen, 2006; Winfree et al., 2007). Commercially managed bees are also available for pollination services and are used in large commercial fields, small gardens, and enclosures such as greenhouses and screen houses. Although the general public gives honeybees much of the credit for pollination, managed bumblebees and solitary bees also have a great impact on certain commodities (Free, 1993; Dag and Kammer, 2001). Furthermore, the use of bumblebees in greenhouses for pollination has become widespread in recent years, and the demand for bumblebees increases every year. Currently, five bumblebee species are reared commercially: Bombus terrestris, B. canariensis, B. lucorum, B. ignitus, B. occidentalis and B. impatiens. In 2004, the estimated number of bumblebee colonies sold was approximately one million: roughly 930,000 colonies of the Eurasian B. terrestris, 55,000 colonies of the North American B. impatiens, and a few thousand colonies of the Eurasian B. lucorum, East Asian B. ignitus, and North American B. occidentalis (Velthuis and van Doorn, 2006).
In general, Bombus species are annual eusocial insects with short-lived colonies, and they are found primarily in the world’s temperate regions. Queens are the only caste of Bombus to overwinter (i.e., enter diapause), and the workers and males perish in the late summer and early autumn, respectively. In the early spring, queens that have overwintered leave their hibernation sites. The queen accumulates a store of pollen and then lays her first batch of eggs into the pollen mass after finding a suitable site for the foundation of a colony. Immediately upon their emergence, the workers of the first brood assume the foraging activities of the queen, who from that point on spends most of her time laying eggs. In late summer, many males and new queens are produced. Only mated queens hibernate and emerge in the spring (Heinrich, 1979, Duchateau and Velthusis, 1988).
In the year-round rearing of B. ignitus bumblebees, one of the key stages is the diapause break. Diapause is defined as a stage in the development of certain animals during which morphological development may be suspended or markedly retarded (Andrewartha, 1952; Mansigh, 1971). The programming of diapause involves the development of specific behavioral, morphological, and physiological design features that uniquely prepare the diapausedestined insect for a period of developmental arrest (Denlinger, 2002). Diapause most likely underlies the variation among queens that may lead to differences in the ability to produce offspring (Beekmand and Vanstratum, 2000). Reduced oxygen consumption is generally regarded as a characteristic phenomenon of insect diapause (Yaginuma and Yamashita, 1999). Studies on oxygen consumption during insect diapause have been performed on eggs of the domestic silkworm (Chino, 1958), Cecropia silkworm (Schneiderman and Williams, 1953), Colorado potato beetles (May, 1989), and other diapause insects (Waku, 1965; Ellingsen, 1978; Crozier, 1979). In the case of insect diapause, exposure to low temperatures is essential for diapause development, which lasts for a fixed minimum duration (Kai, 1983).
With the goal of inducing diapause break, several researchers have attempted to first induce the hibernation of bumblebee queens under controlled conditions, despite the lengthy ovarian diapause of bumblebees (Horber, 1961; Alford, 1969a, 1975; Hoem, 1972; Beekman et al., 1998) and CO2 narcosis (Roseler and Roseler, 1984; van den Eijnde et al., 1991). Under natural conditions, the duration of the hibernation period ranges from 6 to 9 months (Alford, 1969a). Hoem (1972) maintained hibernating B. terrestris queens in mounds of soil in unheated greenhouses or in plastic containers with perlite as bedding; the bees were subsequently placed into a refrigerator at 4-5℃ for 8-9 months. Asada (2004) reported that chilling in a refrigerator at 5℃ for 4 months was effective for inducing nest initiation by B. hypocrite queens. Beekman et al. (1998) demonstrated that weight prior to entering diapause affects the diapause survival of bumblebee queens: queens with a wet weight below 0.6 g were incapable of surviving diapause, regardless of diapause length. Diapausing bumblebee queens require sizeable fat reserves, which are used up during the diapause period, and the amount of metabolic reserves remaining after diapause completion depends primarily on diapause length (Hoem, 1972). Although increased survival rates have been reported in some studies, few studies have attempted to evaluate the effects of different chilling temperatures on the diapause survival rates of queens and their subsequent ability to establish a colony.
To evaluate the effects of chilling temperature on the diapause break of B. ignitus queens, we assessed whether chilling temperature affect the artificial hibernation of B. ignitus queens. The findings indicate that certain temperatures are indeed more favorable for the diapause break of B. ignitus queens.
The insects used in the experiment were 3rd generation queens obtained from B. ignitus colonies reared year-round in a climatecontrolled room (27℃, 65% R.H., and continuous darkness) at the Department of Agricultural Biology, National Academy of Agricultural Science, Republic of Korea.
The basic colony-rearing technique used in the present study was as previously described by Yoon et al. (2002). The queens were reared in three different types of plastic boxes for nest initiation (10.5 × 14.5 × 6.5 cm), colony foundation (21.0 × 21.0 × 15.0 cm), and colony maturation (24.0 × 27.0 × 18.0 cm). The queens were first individually confined in small boxes for colony initiation until oviposition occurred. When the adults emerged from the first brood, the nest was transferred to a medium box for colony foundation and maintained there until the number of workers reached 50. The nest was subsequently transferred to a larger box for further colony development. A 40% sugar solution with 0.3% sorbic acid and pollen dough were provided ad libitum (Yoon et al., 2005). The pollen dough consisted of a sugar solution and pollen (1:1 v:v.)
To examine the effects of chilling temperature on the diapause break of B. ignitus queens, the following environmental conditions were established. The chilling temperature regimes were -2.5℃, 0℃, 2.5℃ and 5℃ under a constant humidity over 80%. After mating for 7 days, mated B. ignitus queens 12 days post-emergence were weighed prior to the experiment. The queens were individually maintained in perlite-filled bottles in a perforated plastic box also containing perlite to prevent mold. These containers were stored in different chilling chambers (under continuous darkness) for 4 months, and the survival rate of the queens was determined monthly. Thirty B. ignitus queens were used in this experiment. At the end of the chilling period, the surviving queens were reweighed. B. ignitus queens stored for 2 months at 2.5℃ or 5℃, which exhibited higher survival rates than queens stored at other chilling temperatures after cold treatment for 4 months, were placed in flight cages for three days (Yoon et al., 2004d). Each queen was subsequently reared in a climate-controlled room (27±1℃, 65% R.H. and continuous darkness). The developmental capacity of each colony was estimated by the preoviposition period, the rate of oviposition, the colony foundation period and progeny-queen production. Queens that did not oviposit within 40 days were excluded from the number of oviposited colonies (Yoon et al., 2004b). The colony foundation period was defined as the amount of time until more than 50 workers emerged from a colony.
The oxygen uptake technique used in the present study was as previously described by Yoon et al. (2004a). The oxygen uptake of B. ignitus queens was measured in a volumetric system with an O2 Uptester (Daiyo Scientific Industrial Co., Tokyo, Japan). B. ignitus queens stored for 3 months at -2.5℃, 0℃, 2.5℃ and 5℃ were used for this analysis. Individual queens were placed in the 20 ml main chamber of each of the vessels. CO2 gas was absorbed into a strip of filter paper saturated with 0.5 ml 20% potassium hydroxide, with the queen separated from the absorbing agent by a porous polyethylene membrane. The oxygen uptake tester was set in a room maintained at 24-25℃, and the chamber containing the queen was submerged in a 25℃ water bath. Readings were taken every 10 min for 1 hr. All of the queens were maintained at 25℃ until they were transferred for measurement. In the case of chilled queens, O2 uptake was measured 1 hr after the queens were transferred to 25℃. Oxygen uptake was expressed as μl/mg body wt./min. Each reported oxygen uptake value represents the mean of 10 replicates.
Statistical analyses were conducted using the chi-squared test and Tukey’s pairwise comparison test (one-way ANOVA) (MINITAB Release 13 for Windows, 2000).
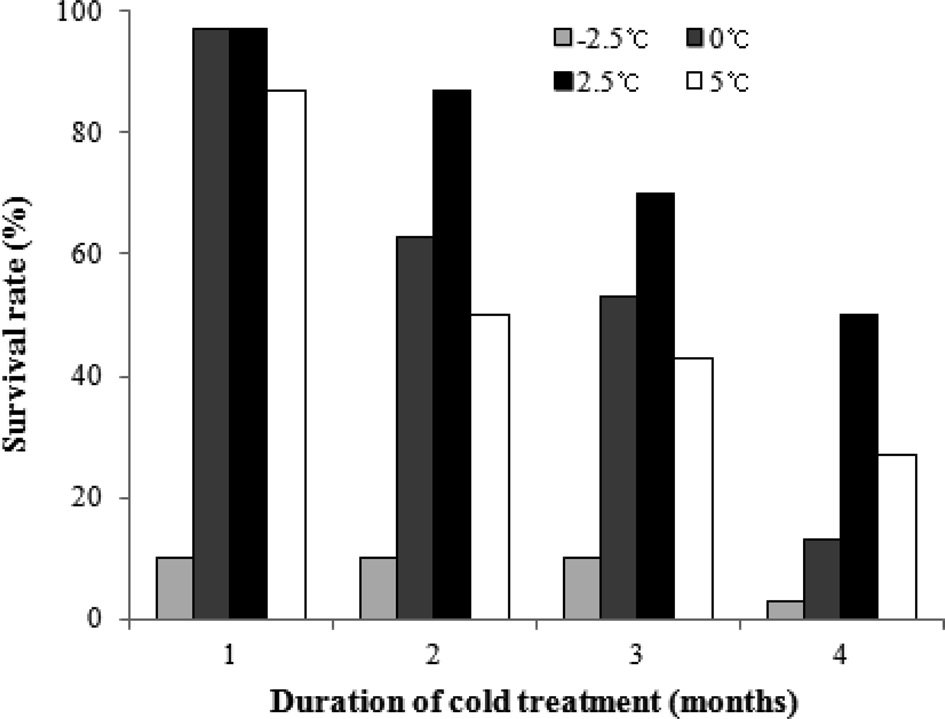
To evaluate the effects of chilling temperature on the diapause break of B. ignitus queens, we investigated the survival rates of queens stored at different chilling temperatures under a constant humidity over 80% (Fig. 1). Comparing the results for the chilling temperature regimes of -2.5℃, 0℃, 2.5℃ and 5℃, the queens stored at 2.5℃ had the highest survival rates, which were 97.0% at one month, 87.0% at two months, 70.0% at three months, and 50.0% at 4 months. The ranking of the remaining temperature regimes in order of decreasing survival rate was 0℃, 5℃ and -2.5℃. The survival rates of B. ignitus queens were statistically affected by the chilling temperature (chi-squared test: x 2=79.373, DF=4, p =0.001 at one month; x 2=37.260, p =0.0001 at 2 months; x 2=23.351, p =0.0001 at 3 months; and x 2=20.497,
p =0.001 at 4 months).
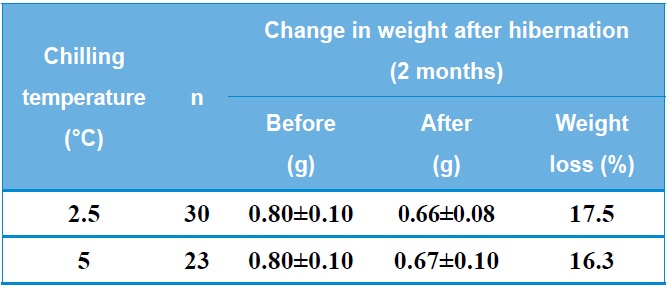
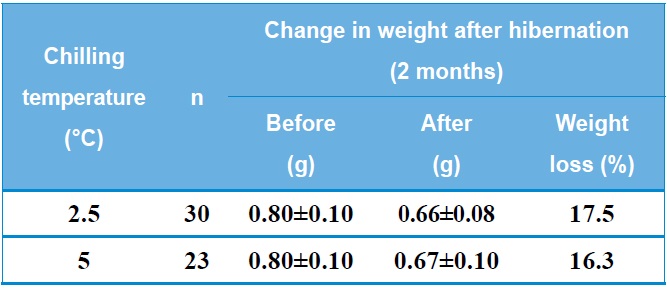
Table 1 shows the change in weight of B. ignitus queens after artificial hibernation at 2.5℃ or 5℃. The rate of weight loss after artificial hibernation for two months was 16.3-17.5%. Horber (1961) reported an average weight loss of 151.3 mg during hibernation and also found that surviving queens were heavier than queens that died during hibernation. Hoem (1972) reported that the observed weight loss occurred during the first half of the hibernation period and that body weight increased after that point. Significant positive correlations were found between the body weight of the queens and the length of survival during and after hibernation. The weight of B. terrestris queens was 400- 1000 mg prior to hibernation and 300-900 mg after hibernation. Similarly, the weight of B. lapidarius queens was 250-850 mg prior to hibernation and 250-750 mg after hibernation. It was also found that the body weight of bumblebee queens was 57% water following hibernation and that both live weight and dry weight were reduced by approximately 50%. Fat makes up an average of 34% of the total dry weight of queen bumblebees prior to hibernation, and 80% of this fat is absorbed during hibernation. Thus, a well-developed fat body is of great importance for the safe hibernation of queen bumblebees (Alford, 1969 a, b). In B. terrestris, survival during diapause is strongly affected by the wet
weight of the queen at the beginning of hibernation. Queens with a low weight (less than 0.6 g) do not survive diapause (Beekman et al., 2000).
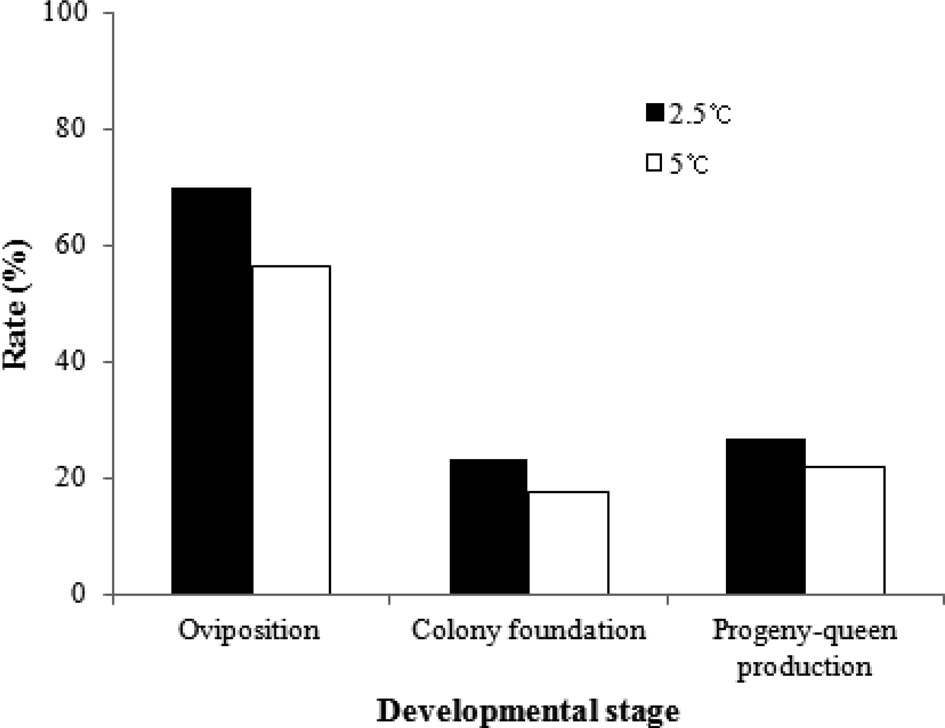
The developmental characteristics of B. ignitus queens stored for 2 months at 2.5℃ and 5℃, which had higher survival rates than queens stored at the other chilling temperatures, were surveyed (Fig. 2). The rates of oviposition, colony foundation and progeny-queen production of queens stored at 2.5℃ were 70.0%, 23.3% and 26.7%, respectively. These values were 1.2- to 1.3-fold greater than those of queens stored at 5℃, but there were no significant differences in the developmental rates of B. ignitus reared post-diapause following artificial hibernation at 2.5℃ and 5℃ (chi-squared test: x2=1.028, DF=1, p=0.311 for
the oviposition rate; x2=0.432, DF=1, p=0.511 for the colony foundation rate; and x2=0.171, DF=1, p=0.679 for progeny-queen production). These results are consistent with the findings of a previous report on B. terrestris (Yoon et al., 2010). Table 2 lists
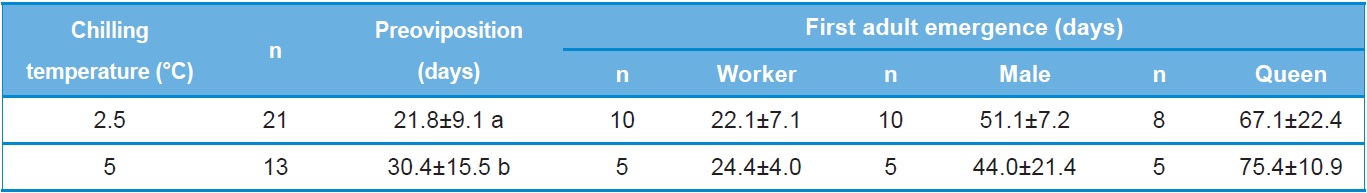
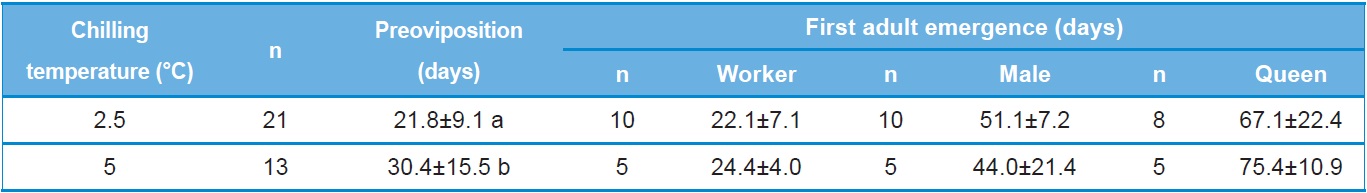
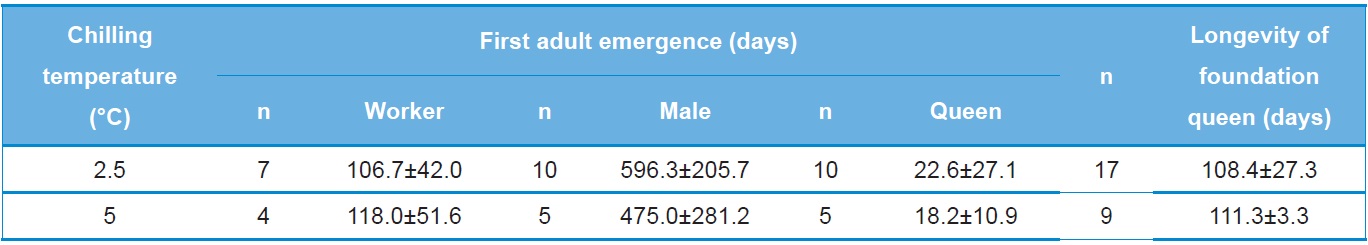
the amount of time prior to preoviposition, colony foundation and first adult emergence of B. ignitus reared after artificial hibernation at 2.5℃ or 5℃. The time to preoviposition of queens stored at 2.5℃ was 21.8±9.1 days, which was approximately 9 day shorter than that of queens stored at 5℃. This difference in preoviposition time was statistically significant (Tukey’s pairwise comparison test: F=4.21, DF=1, 32, p=0.048). No statistically significant difference was detected for the time to emergence of the first workers following storage of the queens at 2.5℃ and 5℃ (F=0.44, DF=1, 13, p=0.518). The time to emergence of the first male for queens stored at 2.5℃ was 51.1 days, which was 7.1 days longer than that of queens stored at 5℃. The time to emergence of the first queen for queens stored at 2.5℃ was 77.1 days, which was 8.3 days shorter than that of queens stored at 5℃. However, these differences were not statistically significant (F=1.76, DF=1, 13, p=0.207 for first male emergence and F=0.59, DF=1, 12, p=0.457 for first queen emergence).
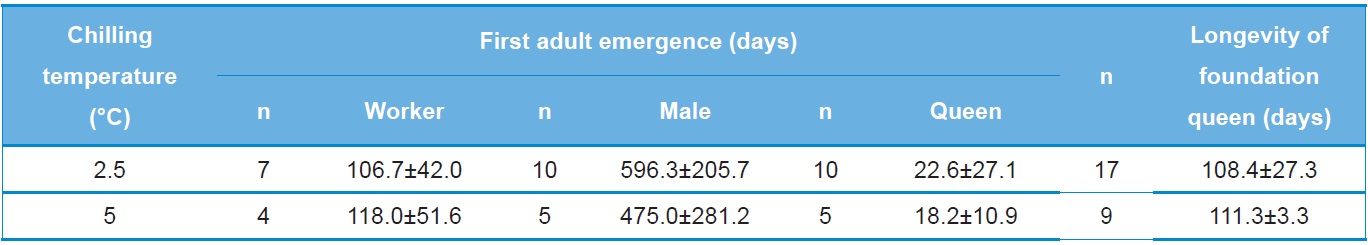
The relationship between the numbers of progeny produced and chilling temperature during diapause was also investigated (Table 3). The number of workers produced by queens stored at 2.5℃ was 106.7±42.0, a value 11.3 lower than that of queens stored at 5℃. The numbers of males and queens produced by queens stored at 2.5℃ were 596.3±205.7 and 22.6±27.1, respectively, which were 121.3 and 4.4 greater than those of queens stored at 5℃. However, these differences were not statistically significant (F=0.15, DF=1, 80, p=0.712 for the number of workers; F=0.76, DF=1, 10, p=0.404 for the number of males; and F=0.04, DF=1, 11, p=0.853 for the number of queens). The longevity of foundation queens stored at 2.5℃ was 108.4±27.3 days, which was approximately 3 days shorter but not significantly different than that of queens stored at 5℃ (F=0.06, DF=1, 24, p=0.806).
Comparing the results for the chilling temperature regimes of -2.5℃, 0℃, 2.5℃ and 5℃, the queens stored at 2.5℃ performed best in terms of survival. The findings indicate that diapause duration is more important for diapause survival than the temperature during diapause and that temperature has no effect on the survival of queens. In contrast to the findings for diapause survival, the preoviposition period is affected by temperature (Beekman et al., 1998).
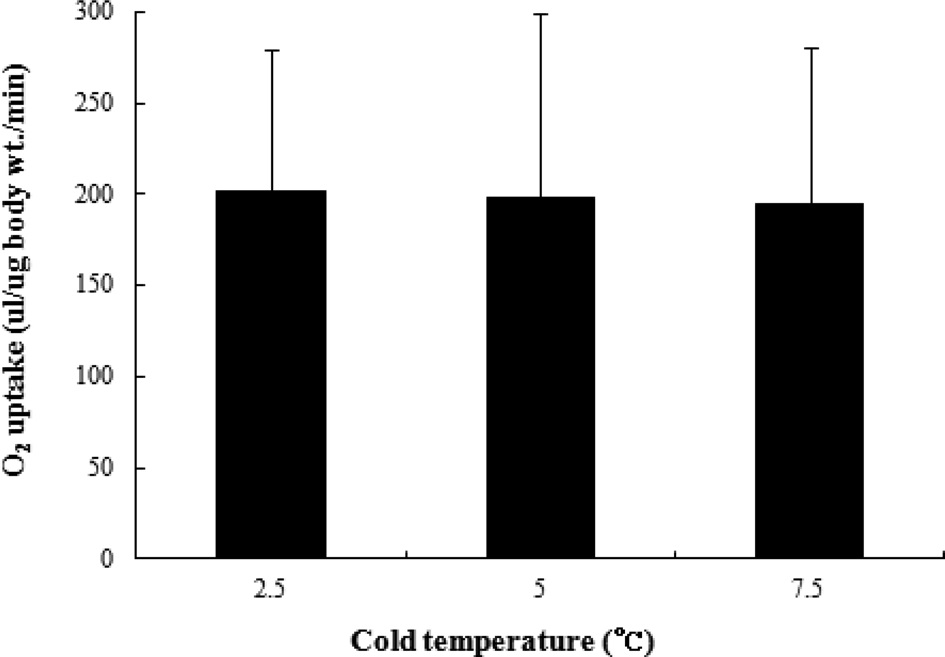
To determine the effect of the different chilling temperatures on oxygen uptake, we measured the oxygen uptake of B. ignitus queens stored for 3 months at various temperatures using an O2 Uptaker. The oxygen uptake values of B. ignitus queens stored for 3 months at 0℃, 2.5℃ and 7.5℃ were 201.7 μl/μg body wt./ min, 198.5 μl/μg body wt./min, and 194.8 μl/μg body wt./min, respectively. Higher oxygen uptake values were generally observed for lower chilling temperatures, but the differences were not statistically significant (F=0.01, DF=2, 35, p=0.9). In silkworm eggs, longer chilling durations have been associated with higher rates of O2 uptake and shorter amounts of time prior to hatching (Yoon et al., 2004c). Kai et al. (1995) (1995) observed that the mode of hatching was also dependent upon the chilling duration. The ability to break diapause has been correlated with increased O2 uptake after transfer to 25℃, and it has been reported that the ability to consume oxygen is recovered over the course of diapause break in eggs exposed to 5℃ (Yaginuma and Yamashita, 1986, 1999).
The results of the present study demonstrate that among the chilling temperatures analyzed (-2.5℃, 0℃, 2.5℃ and 5℃), the queens stored at 2.5℃ had the highest survival rates. Furthermore, the colony developmental characteristics of these queens after diapause were 1.2- to 1.3-fold greater than those of queens stored at 5℃. We therefore conclude that 2.5℃ is an optimal chilling temperature for inducing diapause break in B. ignitus queens.