A number of factors including hormonal, chemical, environmental, physical, behavioural aspects etc. have been attributed to be significant in successful egg deposition by female silk moth (Raabe, 1986). Among them, photoperiod, temperature, humidity, duration, frequency ofmating etc. (Punitham
Keeping this point in view the present study was undertaken to study the reproductive performance of multivoltine and bivoltine breeds and a bivoltine hybrid of silkworm,
Four silkworms breeds/hybrid
The reproductive output of the female moths of different silkworm breeds & hybrid was studied for recording the total number of eggs laid and also the egg retention in the reproductive tract. Finally the data were subjected to statistical analysis. The hatching percentage i.e., number of eggs hatched with respect to total number of eggs laid by the female moth plays an importance role in cocoon productivity for which it was considered during the study.
The ideal condition of silkworm rearing is 25 -28℃ and 70-80% RH. Amongst the four commercial
Reproductive performances of 3 breeds and a hybrid of B. mori L. during different seasons (Values aremean ± SE)
crop seasons of West Bengal i.e. February? March with 20 - 30℃ and 45-75%) and November ? December with 20-30˚C and 45-80 % are considered as favourable seasons, where as , May?June with 32 - 38℃ and 75-95% and September ?October with 30 - 37℃ and 75-98% are considered as unfavourable seasons. Therefore, two favourable and two unfavourable seasons were selected for the experiments.
Multivoltine, Nistari, was selected because it is robust, indigenous and local breed (Kangayam
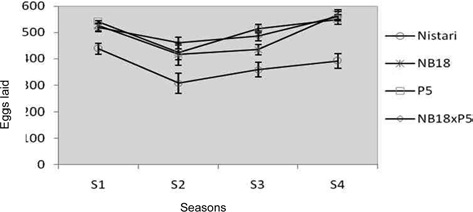
Fecundity and hatching percentage: Reproductive performances of the selected breeds/hybrid studied during different seasons have been presented in Table 1 and the fecundity represented graphically in Fig. 1. The hatching potential of the breeds/ hybrid during the favourable and adverse seasons have been depicted in Fig. 2A and 2B.
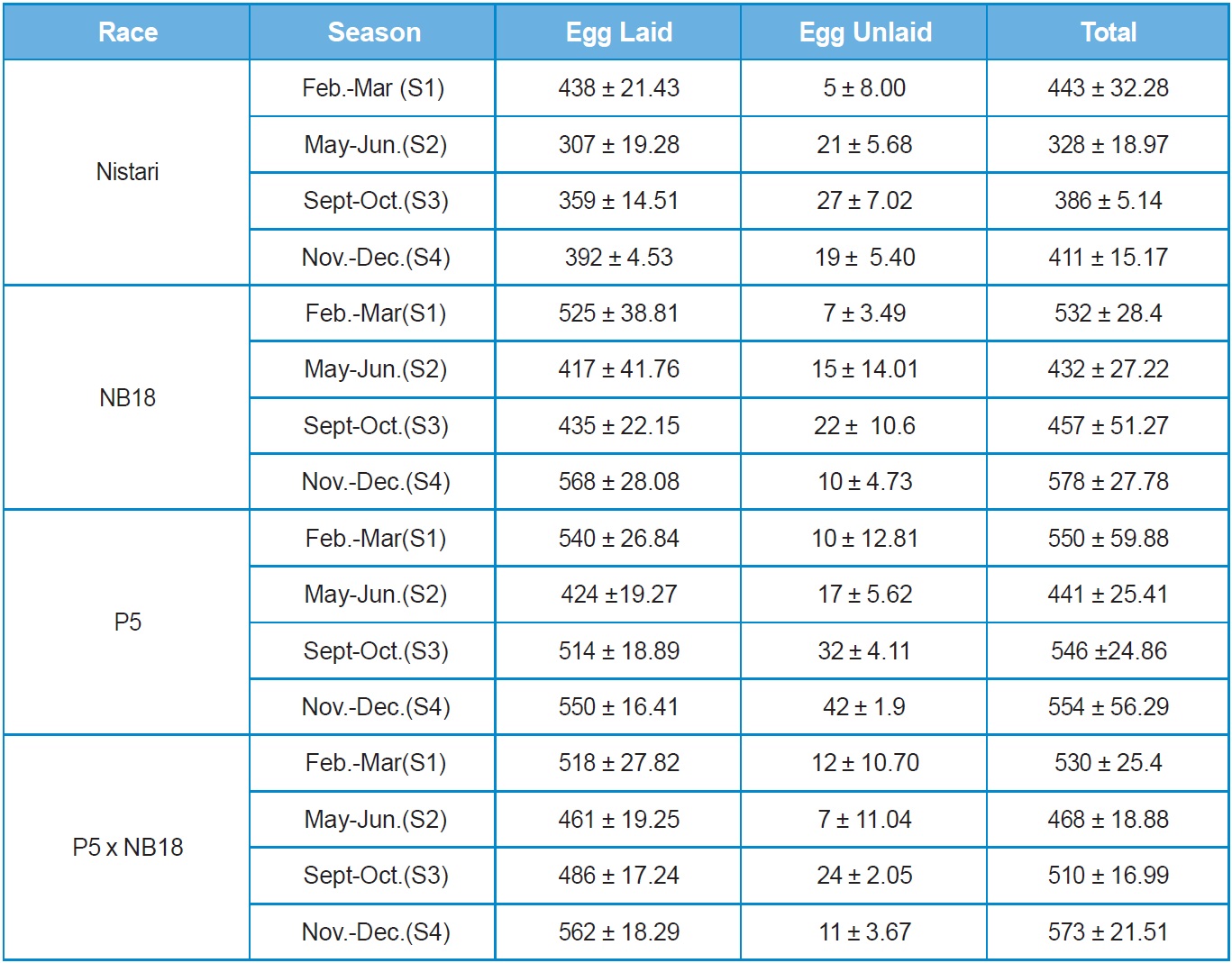
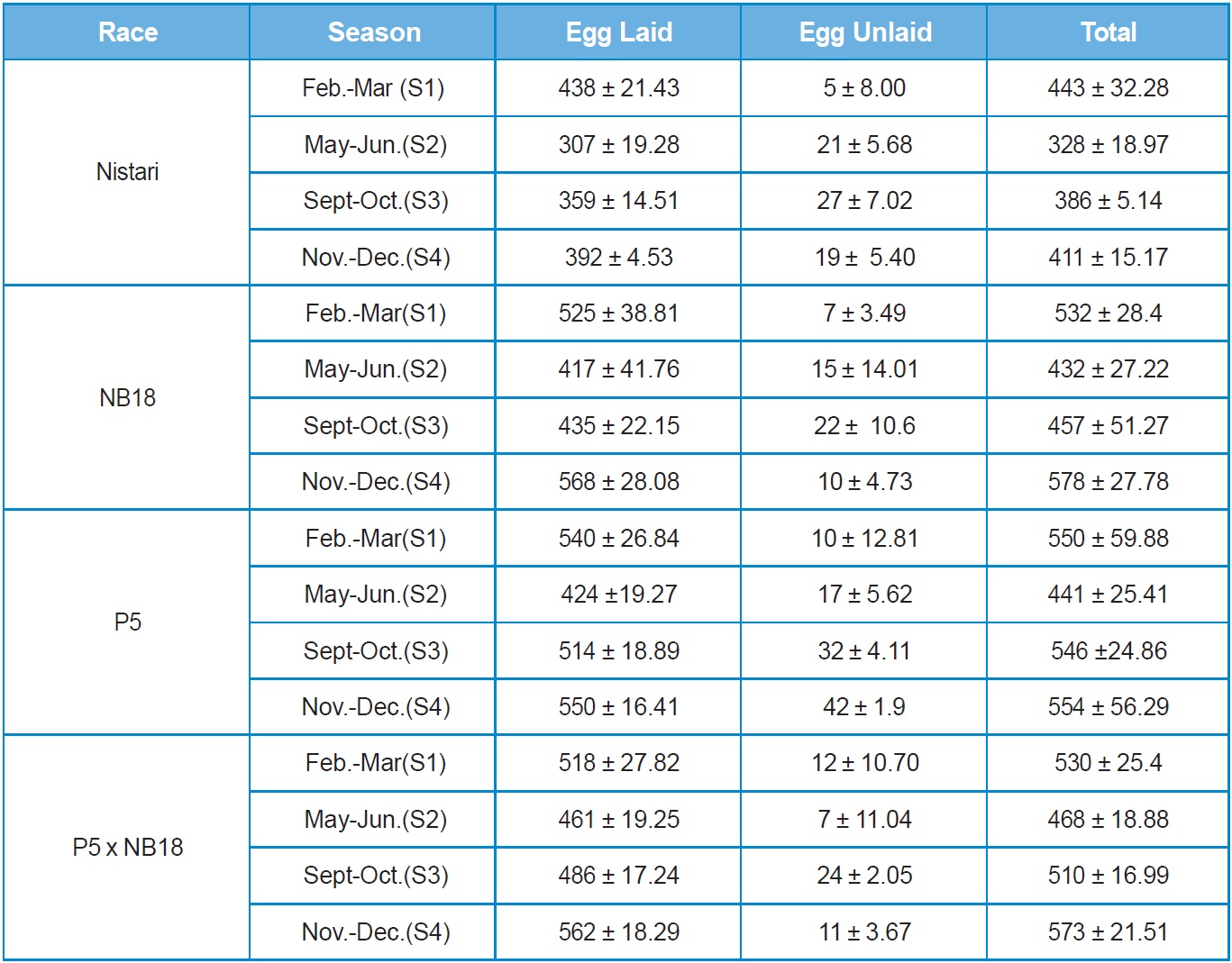
Nistari: In multivoltine breed, Nistari the maximum fecundity of 438 ± 21.43 was recorded in season S1 followed by S4 at 392 ± 4.53. The lowest fecundity
was noted in adverse climatic season of S2 at 307 ± 19.28, whereas maximum egg retention (27 nos.) was recorded in season S3 followed by S2 (19 nos.) and minimum egg retention (5 nos.) was observed in S1.
This clearly demonstrates that climatic conditions during adverse seasons have a greater impact on the race fecundity with maximum egg retention. Total egg production (laid + unlaid) was found to follow the same trend like fecundity. Results on hatching percentage revealed that maximum hatching at 92.33% ± 0.83 was obtained in season S3 followed by S4 at 92.21 ± 2.54 whereas minimum hatching was recorded in season S2 at 83.51% ± 4.33.
NB18: In bivoltine breed, NB18 the maximum egg production was recorded in season S4 at 568 ± 28.08, followed by S1 season at 525 ± 38.81 whereas minimum fecundity was recorded during S2 season at 417 ± 41.70. This was expected to be true in case of bivoltines that generally are difficult to rear during adverse climatic situations as observed in S2 and S3. Eggs unlaid were more in season S3 at 22 nos. and less in S1 season at 7 nos. Total egg production followed the same trend. The maximum hatching percentage of 91.11% ± 4.57 was observed in season S4 followed by almost similar results in season S1 and S3, followed by season S2 at 79.80%± 3.19.
P5: The fecundity and total egg production of another potential bivoltine breed P5 followed the same trend as observed with NB18. Eggs unlaid were found to be more in season S3 with about 32 nos. followed by 17 nos. in season S2. This was followed with season S1 with 10 nos. and season S4 with 4 nos. Although, the hatching percentage was found to be maximum at 91.72% ± 4.72 in season S2 followed by seasons S3, S1 and S4.
P5 x NB18: The hybrid represented a cross between pure lines, P5 and NB18 and therefore had the best fecundity in comparison with the breeds in almost all the seasons reported in the study. There was variations observed in the fecundity and total egg production (laid + unlaid) among the seasons with maximum fecundity of 562 ± 18.29 in S4 season followed by 518 ± 27.82 in S1 season. The lowest fecundity recorded for the hybrid was in season S2 at 461 ± 19.25. The egg retention was found to be the highest at 24 nos. in season S3 followed by S1. However, hatching percentage was recorded the maximum in S3 at 96.75% ± 0.5, followed by season S4 at 90.18% ± 1.83, with the lowest hatching percentage observed in S1 at 86.79% ± 3.45.
Seasonal variation was observed among all the 4 experimental breeds & hybrid, with Nistari showed lesser fecundity in all the seasons compared with the others breeds & hybrid. The highest fecundity was recorded in breed NB18 (568 ± 28.08) compared to other breeds & hybrids in all seasons. While highest hatching percentage was recorded in hybrid NB18 x P5 compared to all other breeds.
The total number of eggs lay therefore associated with the seasons and races, with S1 and S4 being the most favourable season. This confirmed the observations of Tazima (1958), who observed the variation in the number of eggs according to the season and climate. Khan
Egg production depended on the phase relationship between photo- and thermo period and on the frequency of the temperature oscillations (Ratte, 1985). Photoperiod played a phase-setting role in the ovipositional rhythm. In this study, hatching percentage was found to be independent either on changes in environmental condition or silkworm breed and pattern of hatching more or less followed the same pattern in case of all the selected breeds and hybrid. Hatching in mulberry silkworm,
in hatching of
>
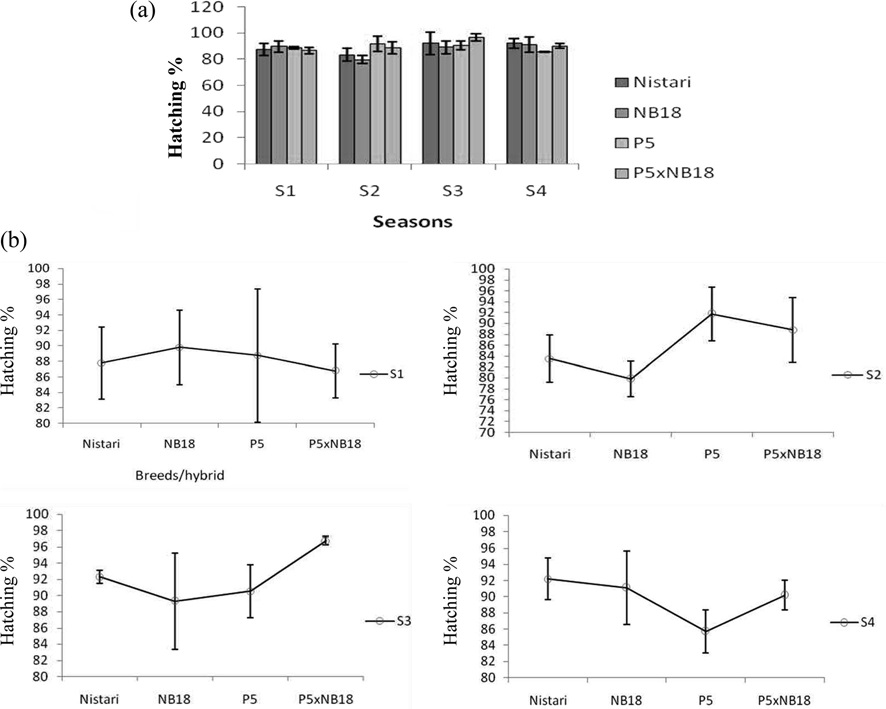
Egg laying rhythmicity after decoupling femalemoth at 9.30 am
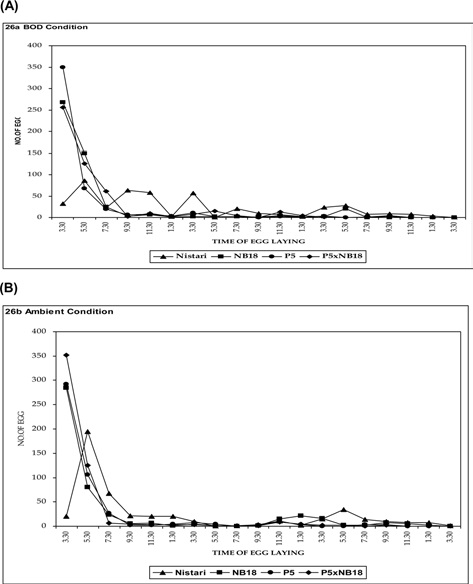
Experiment on egg laying rhythm (the case where the decoupling of the female moth happened at 9.30 am) was conducted both at ambient temperature and BOD conditions and are depicted in Fig. 3A and 3B. Maximum eggs laid were recorded from 5.30 to 7.30 pm in case of multivoltine race, Nistari, whereas minimum egg laid was observed during morning hours. In bivoltine silkworm breeds as NB18 and P5, the egg laying was found to be the highest during afternoon (1.30 pm to 5.30 pm) and minimum during morning hours. The bivoltine hybrid (P5 x NB18) followed the
similar trend of pure bivoltines with maximum egg laying reported from 1.30 pm to 5.30 pm and minimum during morning hours.
>
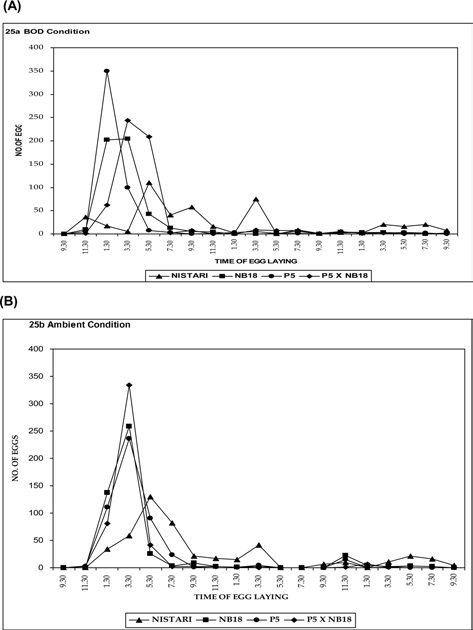
Egg laying rhythmicity after decoupling femalemoth at 3.30 pm
Experiment on the rhythmicity of egg laying (the case where the decoupling of the female moth happened at 3.30 pm) was also conducted both at ambient temperature and BOD conditions and are presented in Fig. 4A and 4B. In multivoltine breed Nistari, maximum egg laid was observed just after decoupling at about 3.30 to 7.30 pm and minimum during morning hours as reported while decoupling the female moth at 9.30 am. In NB18,maximum egg laid was recorded after or at the time of decoupling. In P5 and bivoltine hybrid, P5 x NB18, similar observations were noticed for the egg layings. It was therefore from both the possibilities that egg laying rhythmicity patterns remain more or less committed on similar lines excepting for the differences in oviposition time.
>
Egg laying behaviour and egg production of silkworm, Bombyx mori:
Results of the present investigations also revealed that 28.01% and 66.63% eggs were laid by Nistari during late photoperiod in ambient (room) and BOD conditions respectively when the female moths were allowed to lay eggs at 9.30 am and 20.47% and 33.93% eggs were laid during Scotoperiod in both the conditions respectively, but little or few number of eggs were laid during early photoperiod. The remaining % of eggs was laid in the next late photoperiod in both the conditions. The observations are in conformity with the findings of Fugo (1984). Bivoltines and their hybrid showed that about 90.21% - 95.42% of eggs were laid during late photoperiod in both conditions whereas during Scotoperiod few numbers of eggs were laid.
In another experiment the female moths were allowed to lay eggs at 3.30 pm. 61.57 and 32.26% eggs were laid by during late photoperiod and Scotoperiod. The bivoltines showed a similar pattern of egg laying behaviour like reported previously. Maximum ovulation and oviposition with minimum retention of egg was reported at 25.36 ± 0.17℃ (optimum) temperature and 80 ± 5% relative humidity. Any fluctuation of temperature from optimum level leads to decreased ovulation, oviposition rate, fecundity and increased retention of eggs (Mathur and Lall, 1994).
Logen and Harwood (1965) have reported that in
The study therefore established the potentiality of the hybrid over the pure breeds in laying the eggs in adverse and favourable seasons. It also was proved that congenial climate is a boon as it leads to healthy fecundity with less egg retention and satisfactory hatching percentage, though the variations in some cases may be marginal. The February-March and November - December seasons have been found to provide with good reproductive performance in comparison to adverse seasons. It was also revealed that late photoperiod and early scotoperiod were favourable for egg laid in case of multivoltine breed and bivoltine prefers late photoperiod for egg laying.