Chalcones are naturally occurring, biologically active molecules generating interest from a wide range of research applications including synthetic methodology development, biological activity investigation and studying fragmentation patterns. In this article, a series of chalcones has been synthesized and their fragmentation behavior was studied using modern ambient ionization technique Direct Analysis in Real Time (DART). DART ion source connected with an ion trap mass spectrometer was used for the fragmentation of various substituted chalcones. The chalcones were introduced to the DART source using a glass capillary without sample preparation step. All the chalcones showed prominent molecular ion peaks [M]?+ corresponding to the structures. Multistage mass spectral data MSn (MS2 and MS3) were collected for all the chalcones studied. The chalcones with substitutions at 3, 4 or 5 positions gave product ion peaks with the loss of a phenyl radical (Ph?) by radical initiated α-cleavage, while substitution at 2 position of chalcone in the A-ring gave a product ion peak with the loss of substituted styryl radical (PhCH = CH?). In case of the chalcones with the substituent at 4 positions in A and B rings gave both types of fragmentation patterns. In conclusion, chalcones can be easily characterized using modern DART interface in very short time and efficiently without any cumbersome sample pretreatment.
Chalcones are a large group of phytochemicals that have the general structure of a 15-carbon skeleton, which consists of two phenyl rings attached to the 1 and 3 positions of the 2-propen-1-one moiety with the IUPAC name 1,3-diphenyl-2-propen-1-one (1a). Chalcones are the main precursors for the biosynthesis of flavonoids1 in plants as well as in synthetic organic chemistry. Although flavones were synthesized by the dehydration of chalcones almost a century ago, chalcones are still considered as a subclass of flavonoids. Chalcones are generally synthesized in the presence of methanolic or ethanolic sodium or potassium hydroxide. Various reagents/conditions have been employed for the procedure in the past decade.
Recent studies on biological evaluation of chalcones revealed that some of them possess in vitro and/or in vivo activity as antimalarial, antituberculosis, antitumor, antileishmanial, anti-inflammatory, antimitotic, nitric oxide inhibitors, antihyperglycemic, NADH: ubiquinone oxidoreductase inhibitors, hyperglycemic, glucosidase inhibitor, Antidiabetic, antiulcerogenic and as phase II metabolic enzymes inducers, etc.
Because of the intriguing structure and importance of chalcone a number of research group have been involved in structural characterizations and/or studying their fragmentation patterns in the last several decades using mass spectrometry. Recently, Zhang
Recently, DART has been used for the chemical profiling of the different landraces of Piper betle leaves,9 for quality and authenticity assessment of olive oil,10 in the detection of breast cancer,11 screening for pesticides,4 serum metabolomic fingerprinting,12 multiple mycotoxins in cereals,13 in the analysis of purified pharmaceutical preparation using thin-layer chromatography,14 and most recently in the analysis of phthalates added to food and neutraceutical products.15 The technique has also been recently reviewed.16,17 To the best of our knowledge, ambient ionization technique DART has not been used as the ion source for the mass spectral analysis of organic small molecules. In this study, we have synthesized a series of chalcone derivatives and studied their fragmentation pattern using DART ion source in an ion trap mass spectrometer. DART is fast, simple, but can detect trace chemicals in complex matrices, using minimal or no sample preparation.
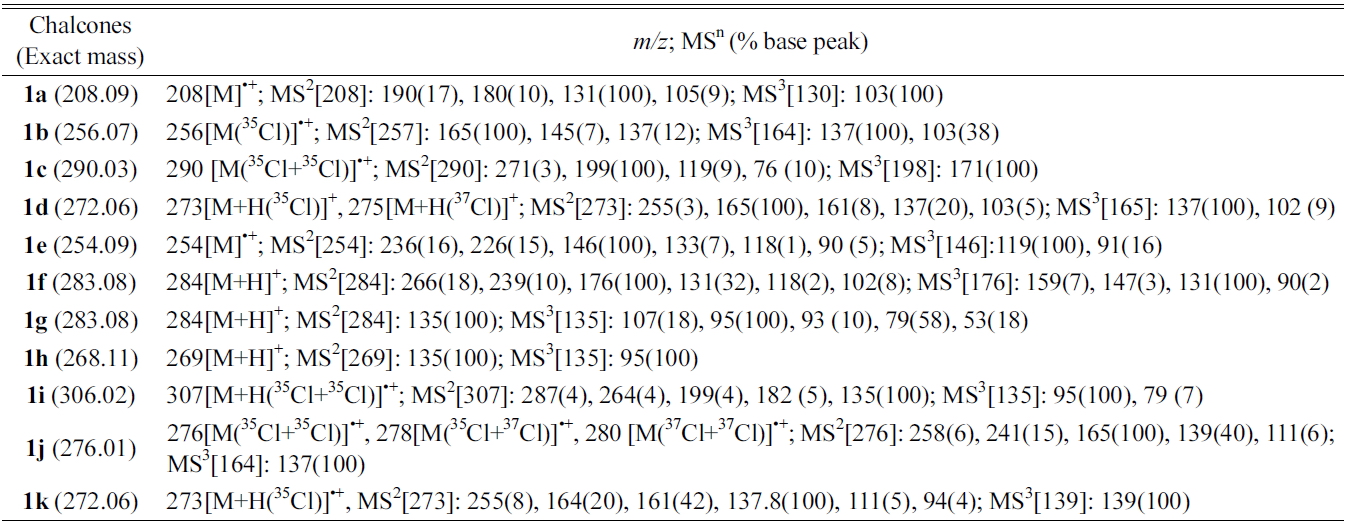
[Table 1.] Multistage MSn data of chalcones by DART ion trap mass spectroscopy
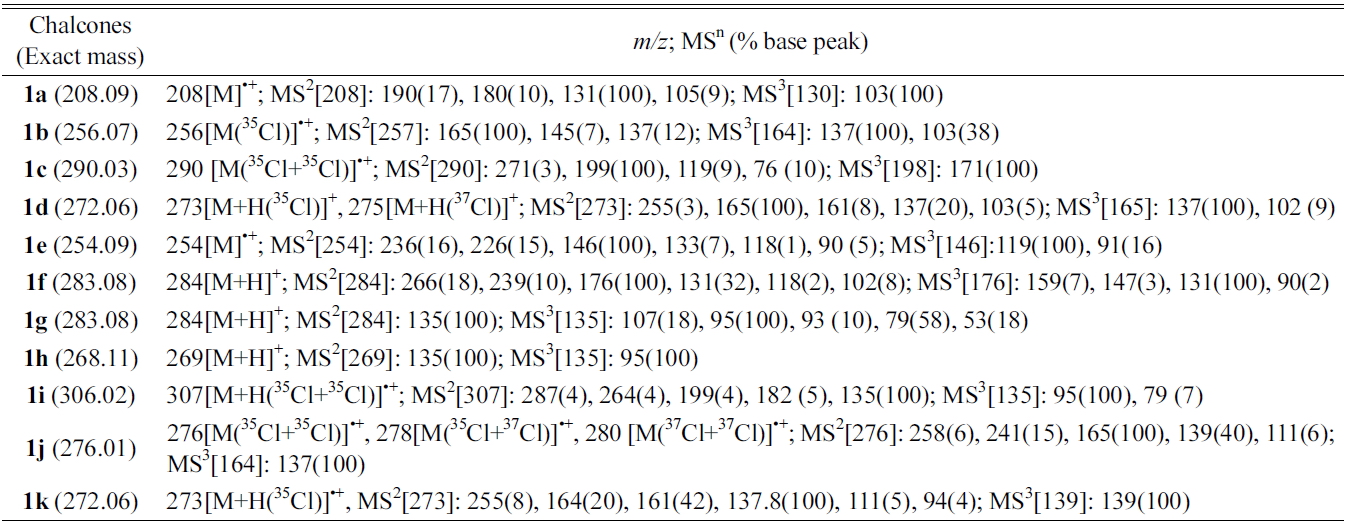
Multistage MSn data of chalcones by DART ion trap mass spectroscopy
Benzaldehyde and acetophenone derivatives were purchased from Alfa Aesar and Sigma-Aldrich and were used without further purification. Reagents and solvents were analytical grade and used without distillation.
All experiments were performed using an ion trap mass spectrometer from Agilent technologies with an IonSense (Saugus, MA) Direct Analysis in Real Time (DART) ionization source. For the DART source, helium gas was used at a flow rate of 4 L/min. The gas heater and capillary voltage of the DART source were set to 350℃ and 4000 V, respectively. The distance between the outlet of the DART and the inlet of the orifice of the mass spectrometer was set 1 cm. Sample introduction was accomplished by slowly moving the closed end of a glass capillary, which was dipped into powdered analytes so that sample was carried across the helium gas stream between the DART source and the orifice of the mass spectrometer. The spectra recording interval was 0.5 s. Fragmentation was carried out with the following parameters: trap drive 78, ICC on, accumulation time 200,000 s, and fragmentation amplitude was 1 volt.
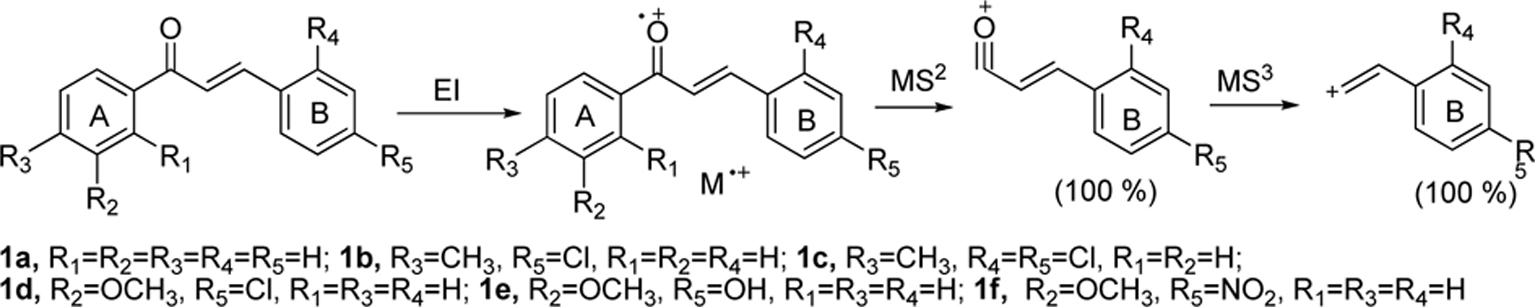
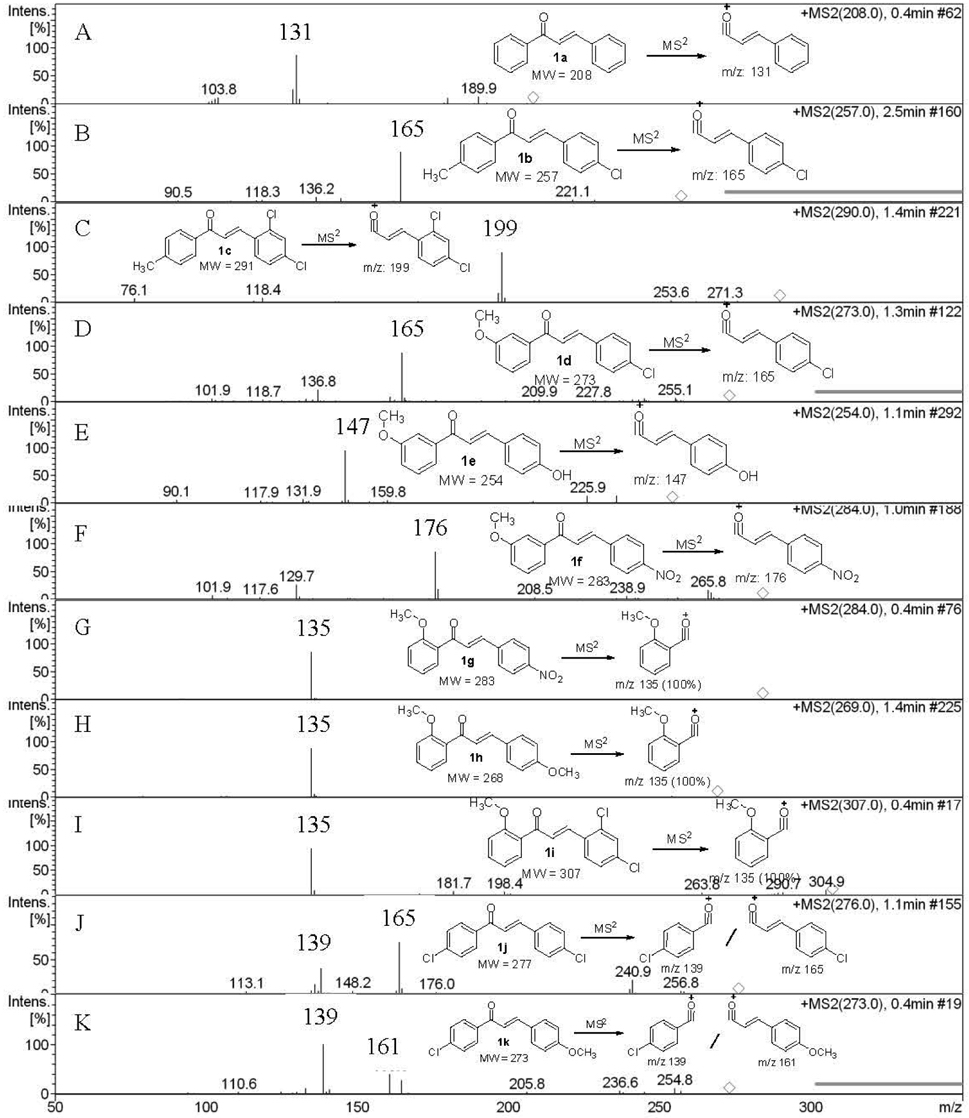
Chalcones were synthesized using a mixture of (substituted)-benzaldehyde and (substituted)-acetophenone in ethanol was added alcoholic sodium hydroxide (10%) and was stirred for 2-3 hours and obtained quantitative yields of the corresponding chalcones. The solid chalcones were introduced into the DART source without any other sample preparation. The chalcones tested gave molecular ion peak [M]?+ and are summarized in Table 1. For example, chalcone 1a (without substitutions in the A or B rings) having molecular weight 208.15 (nominal mass 208) gave molecular ion peak at
Multistage mass spectral data (MS2and MS3) were taken for all the chalcones synthesized and are also summarized in Table 1. MS2 of chalcone 1a gave
Product ion peaks together with their corresponding proposed structures obtained from MS2 for all the chalcones 1a-f are shown in Figure (1A-1F). For all the cases, chalcones 1a-f gave product ion peaks with the loss
of substituted phenyl radical via α-cleavage. Substituent at 3 or 4 positions of the A ring and 2' and 4' positions of B ring did not affect the product ion peaks. In addition, MS2 of chalcone 1a gave three more ion peaks at
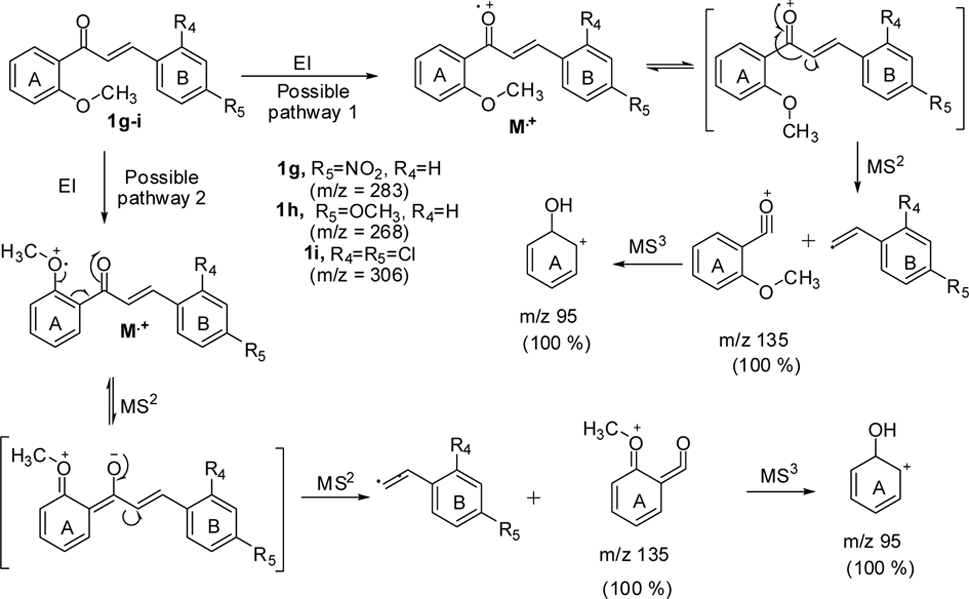
With substitution at position 2 of the A-ring of chalcones 1g-i, the MS2 fragmentation gave
As shown in Scheme 2, all three compounds (1g-i) have shown the product ion peak at
metastable ion peak for the chalcones 1g-i and their corresponding structures are shown in Figure (1G-1I).
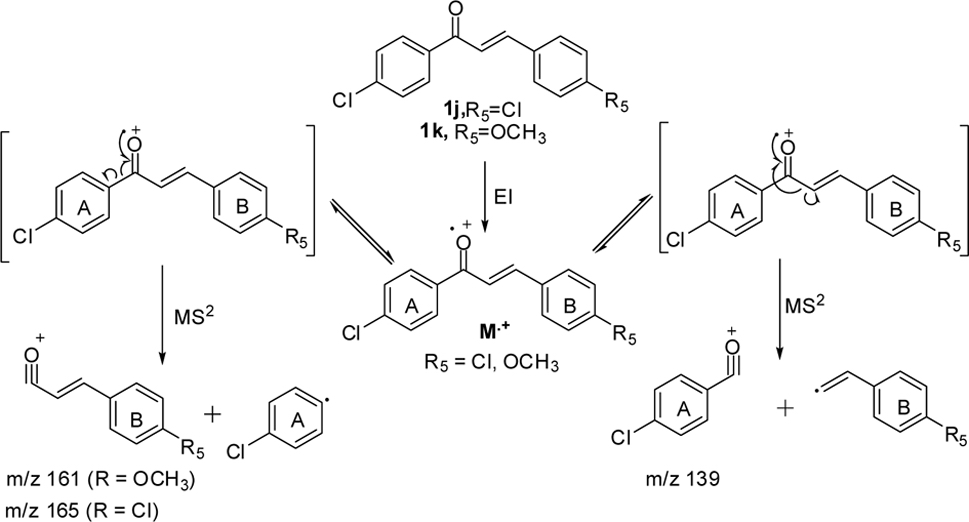
The effect of the substituent was observed in DART while examining the chloro-substituted chalcone (1j) at 4-position of the A-ring (Scheme 3), two peaks corresponding to the product ions were obtained, one of which was at
In addition, MS2 of 1j gave
The fragmentation behaviors of Chalcones were studied using very convenient ambient ionization technique DART in combination with a mass spectrometer and found all the chalcones gave similar fragmentation pattern with the molecular ion peak as [M]?+ . This method is easy, convenient, time saving and most importantly the samples can be analyzed at their natural states without sample preparation. Substitute in A ring at 2' position gives product ion peak with A ring; Substitute in B ring at any positions gives product ion peak with B ring; Substitute in both A and B rings at 4 positions gives two product ion peak with A and B rings, respectively. Taken together, we can conclude that, chalcones can be easily characterized using modern DART ion source in very short time and efficiently.