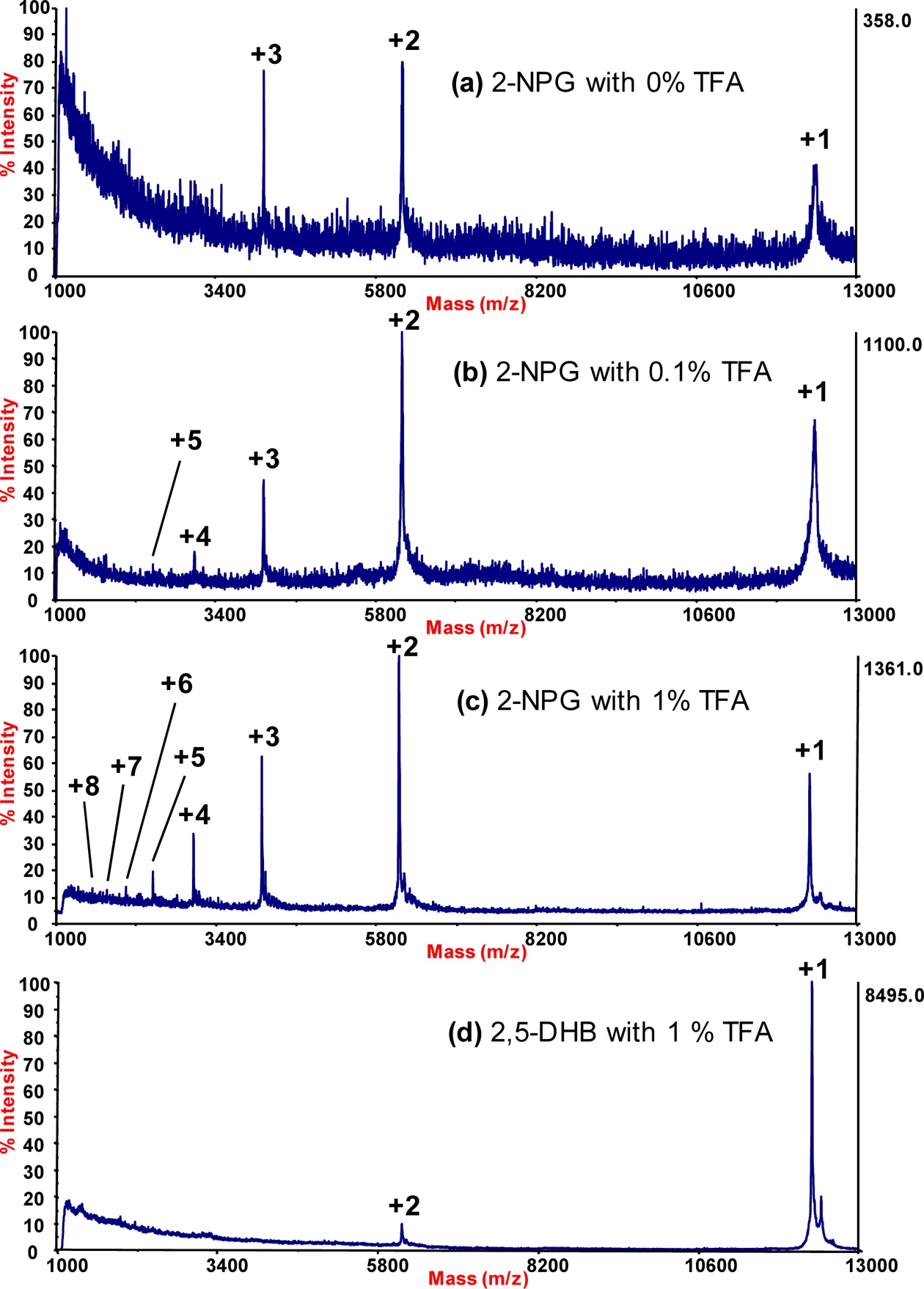
The effects of trifluoroacetic acid (TFA) were evaluated on the generation of multiply charged ions of cytochrome c in a 2-nitrophloroglucinol (2-NPG) matrix in high-vacuum, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). The presence of 1% TFA in the 2-NPG matrix solution was more effective in generating multiply charged protein ions than matrix solutions containing 0.1% or 0% TFA. Regarding the matrix itself, with 1% TFA, 2-NPG was significantly more effective in generating multiply charged ions than 2,5-dihydroxybenzoic acid (2,5-DHB). The maximum charge state of cytochrome c was +8 when using a 2-NPG matrix containing 1% TFA.
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) is a soft ionization technique frequently used for the analysis of proteins. MALDI matrices are effective energy mediators that facilitate analyte ionization. In sample preparations for MALDI-TOF MS, the analyte is dispersed in the matrix solution and deposited as a single sample spot. A pulsed laser beam then strikes the sample spot, causing desorption of both the matrix and analyte. MALDI-TOF MS typically provides a mass spectrum of mostly singly charged proteins in a vacuum system, while electrospray ionization MS typically yields multiply charged protein ion peaks.1,2
Generally, conventional matrices such as 2,5-dihydro- xybenzoic acid (2,5-DHB), sinapic acid, and α-cyano-4-hydroxycinnamic acid generate mostly singly charged protein ions in high-vacuum MALDI systems. Recently, however, atmospheric pressure MALDI reportedly can produce multiply charged protein ions under certain conditions.3 This technique is referred to as laser spray ionization (LSI). More recently, the employment of a new matrix, 2-nitrophloroglucinol (2-NPG), was shown to yield multiply charged protein ions even in high vacuum.4 Since most commercial MALDI-TOF MS instruments operate in high vacuum, the production of multiply charged protein ions, facilitated by the 2-NPG matrix, would significantly reduce the
The current study investigated the efficiency of generating multiply charged protein ions under high-vacuum MALDI-TOF MS conditions using 2-NPG as a sample matrix, where various levels of trifluoroacetic acid (TFA) were added to the sample matrix to enhance and optimize the generation of multiply charged protein ions. This is the first systematic investigation on the effects of acid additives in a MALDI sample matrix on the generation of multiply charged protein ions.
2,5-DHB, 2-NPG, TFA, acetonitrile (ACN), and horse heart cytochrome
All mass spectra were obtained on a Voyager MALDI-TOF mass spectrometer (Applied Biosystems, Foster City, CA, USA) equipped with a nitrogen laser (337 nm, 3 ns pulse, 20 Hz repetition rate) in linear ion mode for a mass range of 500~15,000 Da.
To prepare the 2,5-DHB matrix solutions, 10 mg of 2,5-DHB was dissolved in 1 mL of aqueous 50% ACN/1% TFA. The 2-NPG matrix solution was prepared by dissolving10 mg of NPG into 1 mL of aqueous 50% ACN, 50% ACN/0.1% TFA, or 50% ACN/1% TFA solutions. To make a protein stock solution, 10 mg of cytochrome
A dried-droplet sample preparation method was adopted for MALDI-TOF MS analyses. A 1-μL droplet of the mixturesolution (matrix:sample = 1:1 by volume) was placed on a MALDI plate and allowed to dry, forming a microcrystal layer.
Molecular structures of 2-NPG and 2,5-DHB are shown in Figure 1. Note that 2-NPG is a novel matrix used to enhance the generation of multiply charged protein ions in high-vacuum MALDI-MS analysis. 2,5-DHB is one of the most commonly used matrix materials in MALDI-MS analyses.5
Figure 2 shows MALDI mass spectra of cytochrome
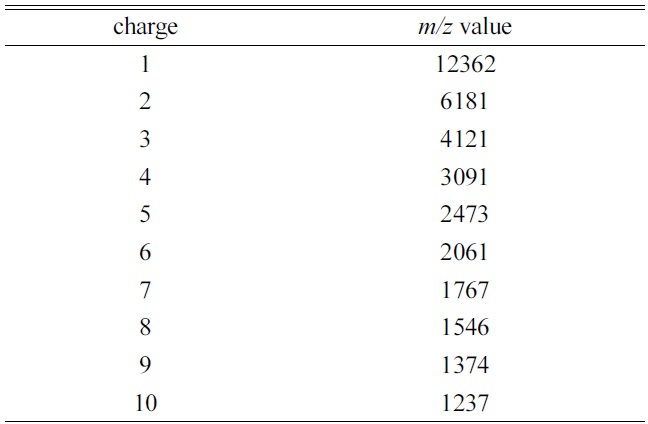
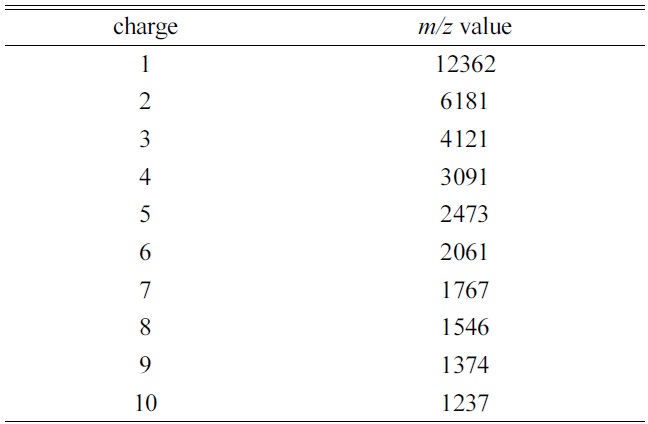
[Table 1.] The theoretical average m/z value for each charge state of cytochrome ca)
The theoretical average m/z value for each charge state of cytochrome ca)
in Table 1. The MALDI mass spectrum of cytochrome
Higher concentrations of TFA (> 1%) were also evaluated, but no improvements over the performance of matrices containing 1% TFA were observed. The 2-NPG matrix containing 1% TFA provided the highest abundance of multiply charged cytochrome
Trimpin and coworkers4 were the first to report the use of a 2-NPG matrix to enhance the generation of multiply charged protein ions in high-vacuum MALDI analyses. However, no mention was made as to the amount of acid, if any, added to the matrix solution. A 2-NPG matrix in 50:50 ACN:H2O was also used without acid in a recent high-vacuum application of LSI.6 The current analysis demonstrates that the addition of a small amount of TFA (1.0%) to the 2-NPG matrix solution can significantly improve the detection of multiply charged protein ions.
The generation of multiply charged protein ions using a 2-NPG matrix containing TFA was evaluated and optimizedin a high-vacuum MALDI system. A 2-NPG matrix containing 1% TFA was optimal in terms of signal-to-noise and peak abundance for creating multiply charged protein ions. These results are a significant improvement over analogous analyses using a 2,5-DHB matrix. The generationof multiply charged protein ions shifts larger molecules into a lower