An experimental study on the nascent product of the OH heterogeneous reaction with NaCl was performed under dry and wet conditions using a bead-filled flow tube system coupled to a high-pressure chemical ionization mass spectrometer. The ozone concentration in the flow tube for the atomic hydrogen removal was varied in order to control the conversion reaction of molecular chlorine into HCl for the identification of the nascent product. The mass spectrometric observation was that the O3 introduction reduced the concentration HCl, while it increased the concentration of Cl2 and ClO. Based on the experimental results, we suggest that the nascent product of the titled reaction is gaseous Cl2, which is followed by fast conversion into HCl in presence of H. No significant difference in the concentration profile between under dry and wet (RH = 2%) conditions was observed.
As a key oxidant, the hydroxyl radical (OH) is involved in a variety of reactions in the atmosphere. Although the main sinks of OH in the atmosphere are gas-phase reactions, such as the reactions with carbon monoxide, methane, or biogenic hydrocarbons, heterogeneous reactions have been suggested as additional sinks in order to explain the overestimation of OH concentration in the atmospheric models. According to the kinetic studies on the heterogeneous reactions of OH, the reactions with organic aerosol matters, such as paraffin wax, stearic-palmitic acid, pyrene, and soot, are relatively fast, while those with inorganic compounds including salt, water, ice, and sulfuric acid are slow.1-4 However, recently it is reported that the heterogeneous reaction of OH with sea-salt is enhanced under wet conditions,56 which is a probable case in the coastal area.
The OH reaction on sea-salt aerosols plays an important role in the production of chlorine, which is responsible for further reactions in the troposphere and the stratosphere, for example, ozone destruction. Since atomic chlorine (Cl) is an extremely powerful oxidant with the reactivity one or two order-of-magnitude higher than that of OH,7 considerable attention has been paid to the heterogeneous reaction of OH on the sea-salt, such as NaCl, MgCl2, and CaCl2. Recently, we observed chlorine-containing ions, which are HClF- (55 amu) and SF5Cl? (162 amu), in the flow tube study on the OH heterogeneous reaction with NaCl using chemical ionization mass spectrometry (CIMS). Those ions are likely the products of the ion transfer reaction from
to HCl and atom exchange reaction between
and HCl, respctivley (see Reaction 1 and 2).8
However, the origin of HCl is not yet certain. It is also possible that the OH heterogeneous reaction with NaCl induces the release of HCl directly from the OH uptake surface. In addition, molecular chlorine (Cl2) should not be excluded as the product of the reaction since HCl can be formed from the irreversible conversion of Cl2 right after release from the OH uptake surface in the presence of atomic hydrogen (H), with the extremely high reaction constant (2.0 × 10?11 cm3 molecule?1 s?1).9
A considerable amount of atomic hydrogen may be present in the flow tube as a residue, even after the gas phase reaction of H and O2 that were supplied as the OH production sources. Therefore, identification of the nascent product of the OH heterogeneous reaction with NaCl by
selectively inhibiting one of the two pathways is required in order to understand the mechanism of the reaction and to improve the atmospheric model. In this regard, ozone (O3) is the best inhibitor of Reaction 3, as it scavenges H very well with a high reaction constant, 3.0 × 10?11 cm3 molecule?1 s?1.10
On the other hand, the removal of HCl and Cl2 by O3 is negligible; the kinetic constants for O3+HCl and O3+Cl2 are 4.7 × 10?24 and 3.0 × 10?28 cm3 molecule?1 s?1, respectively.1112
In this study, we monitored HCl, Cl2, and ClO as introducing O3 into the flow tube system in which the OH heterogeneous reaction with NaCl was taking place. Hydrogen atoms remaining even after the OH production was scavenged through O3 purging, which removed the possibility of the HCl production through the reaction with Cl2. The mass spectrometric observation of the ion species provides us information as to what the nascent product of the heterogeneous reaction of OH with NaCl is, which is important in the atmosphere in the coastal area.
Experimental studies were performed under flow conditions at 100 Torr and room temperature using an experimental setup described elsewhere.56 There were two modifications made in order to enhance the sensitivity: (1) an increased number of beads (80 instead of 60) that was used to pack the reactor tubes, (2) no pre-pumping before the chemical ionization (CI) region, which allows all species in the flow tube to proceed to the CI region without any loss. The flow-tube was coupled to a high-pressure chemical ionization mass spectrometer equipped with a quadrupole analyzer and an electron multiplier.
The reaction H + O2 + M was used to produce OH radical, which induced heterogeneous reactions on the NaCl surface at 100 Torr. A molecular hydrogen flow was diluted by helium (Airgas, UHP), and was then discharged inside a Beenaker microwave discharge cavity operating at 30 W to produce hydrogen atoms which then react with O2 (Matheson Tri Gas, 99.5%). Ozone was generated from O2 with an ozonizer (ORTEC), trapped in silica gel at ?70℃, and then was evaporized at room temperature into a glass bulb to prepare a O3/He mixture. The mixture of Cl2/He was prepared by dilution of He and gaseous Cl2 (Matheson Tri Gas, 99 %).
Gas flow rates were monitored with calibrated electronic mass flow meters (Tylan), and pressure in the differential flow-tube was measured with an absolute pressure gauge (MKS 1000, Baratron). Glass beads of 3 mm diameter were coated with NaCl (Mallinckrodt, 99.9%) using the techniques described previously.56 The parent ion of CIMS,
, was ionized using a radioactive polonium source (NRD, 210Po). The CIMS sensitivity of O3, OH, and HCl were 7.7 × 107, 4.2 × 107, 3.7 × 107 molecule cm?3 cps?1, respectively.
No reference tube was used as the uptake coefficient was not calculated, which is based on the additivity of kinetic resistances.13 The experimental setup with the gas flow configuration used in this work is schematically described in Figure 1.
To verify the reproducibility of Reaction 3 and Reaction 4 in our system, a gaseous flow of Cl2 was introduced into the empty flow tube as well as with H2, both of which were diluted in a carrier gas of He/N2. The concentration of H was assumed to be proportional to the concentration of H2 that passed through the microwave discharge. The mass peaks derived from HCl, i.e., HClF? (m/z 55 amu) and SF5Cl? (m/z 162 amu), were not observed until H was produced upon ignition of the microwave discharge.
As shown in Figure 2(a), the concentrations of HCl and Cl2 are inversely proportional to each other. The inhibition of Reaction 3 by Reaction 4 was also confirmed by introducing O3. As the amount of O3 introduced increased, HCl produced from the reaction with Cl2 decreased, as can be seen in Figure 2(b). This is because O3 depleted H that could react with Cl2. However, when H atom production is inhibited by turning off the microwave discharger, the concentration of Cl2 did not change even when O3 was introduced, which confirmed negligible interference of Cl2 by O3 itself.
Gaseous HCl, Cl2, and ClO were observed under dry and wet conditions, when OH was present at the initial concentration of approximately 1012 molecule cm?3. As discussed previously in detail,56 OH radical in the gas phase reacts with NaCl on the surface of glass beads. Although the mechanism for this reaction is still under discussion, Cl2 is considered as the most probable product of the reaction.1415 However, the observations made in the current study revealed that the dominant species of chlorine compounds was HCl, while only a trace amount of Cl2 was detected. More care must be taken to conclude that HCl was the dominant reaction product. A nascent chlorine molecule that was generated from the above-mentioned reaction might have been transformed immediately into HCl, HOCl, and ClO in the presence of H, OH, and O through some unidentified heterogeneous reactions.
It should also be noted that considerable amounts of H, OH, and O present in the system originated from the OH production.
Chlorine monoxide (ClO) was also produced from Reaction 5 (k = 4.3 × 10?14 cm3 molecule?1 s?1).15
Atomic chlorine was not observed, due to its reaction with H2, OH, and HOCl (k = 1.8 × 10?14, 7.1 × 10?16, and 1.2 × 10?12 cm3 molecule?1 s?1, respectively).1617
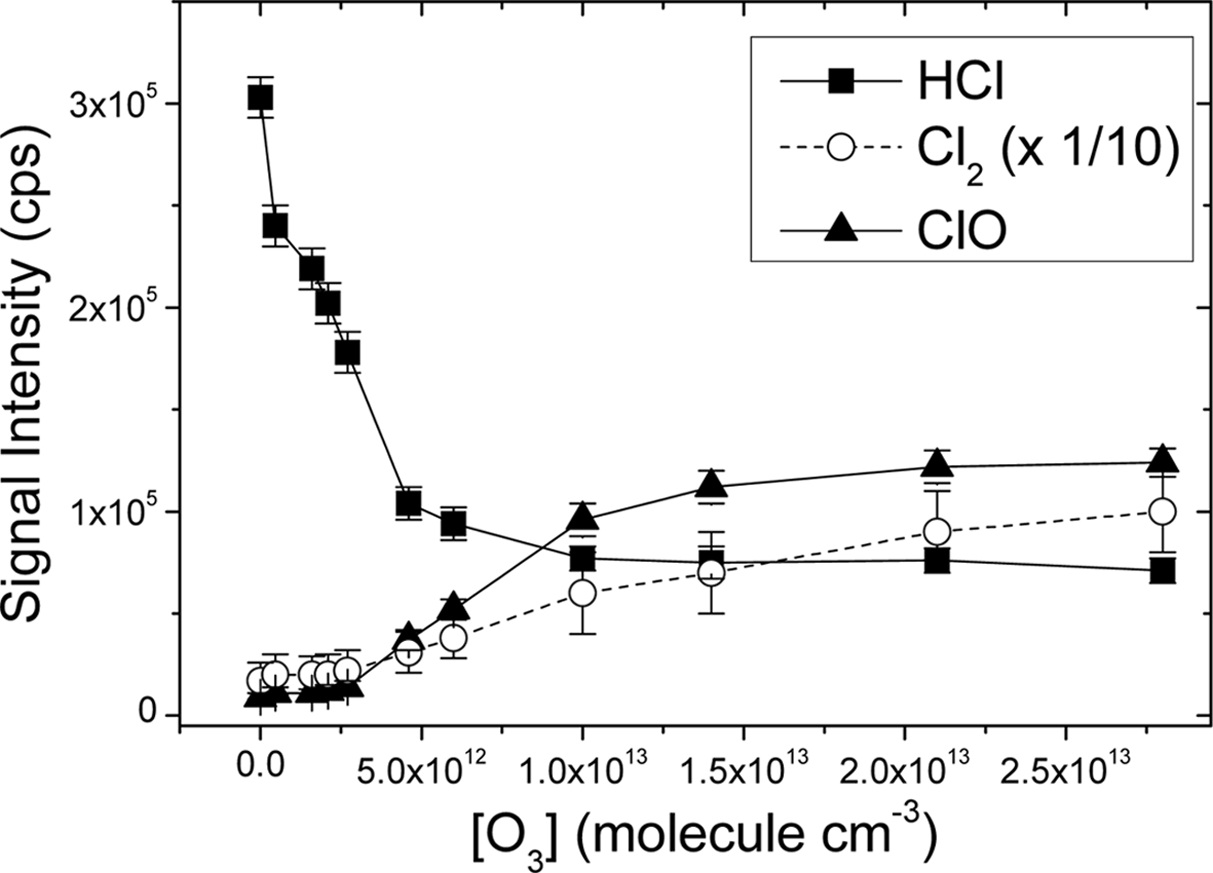
Figure 3 shows that the HCl concentration monotonically decreased as Reaction 3 was inhibited by the quenching reaction with O3 under the dry condition. The HCl reduction leveled off at the O3 concentration of approximately 1.0 × 1013 molecule cm?3. On the other hand, the Cl2 concentration increased at higher [O3] although the change was not as clear as the HCl concentration. As mentioned in the introduction section, this implies that the observed HCl is not the nascent product of the titled reaction, but the conversion product of Cl2 in the presence of H. Otherwise, [HCl] with respect to [O3] would have given no change as the reaction of O3 + HCl is extremely slow, which is contrary to our observation.
The assumption that Cl2 is the nascent product is additionally supported by the observation that the production of ClO increased by one order-of-magnitude at [O3] = 1.0 × 1013 molecule cm?3 (see Figure 3). Due to the considerable difference in the rates between Reaction 3 and 5, Cl2 has little chance to react with O in the presence of H. However, Reaction 5 becomes more probable when the removal of H by O3 occurs, resulting in enhancement of the ClO production. The build-up of ClO leveled off when the reduction of HCl began to slow down.
The concentration changes of HCl, Cl2, and ClO in the flow tube as the added O3 varied were also monitored under a slightly wet condition (RH = 2 %). The relative humidity (RH) was kept to only 2% since no pre-pumping was given in front of the CI region in order to prevent the loss of detection sensitivity. The concentration changes were basically the same as those at dry condition although all the signal intensities of the species were reduced by the factor of ~3 due to the loss of the parent ion
by complex formation with H2O. This implies that Cl2 is more likely to be the nascent product of the titled reaction than HCl at 2 % of RH or less.
It must be noted that further studies are required to conclude the identification of the nascent product of the titled reaction since the reaction system of the flow tube in this study was too complicated. One of the possible studies will be the computational modeling which can enable us to cover a wide variety of reactions that possibly occur in the flow tube system but evade experimental investigation. A comparison of the modeling results with those from experimental studies is expected to provide valuable information on the nascent product of the titled reaction.
An experimental study to identify the nascent product of the heterogeneous reaction of OH with NaCl surface was performed under dry and wet conditions using a bead-filled flow tube system coupled to a high-pressure chemical ionization mass spectrometer. The gaseous OH radical generated in situ through H + O2 + M reaction reacts heterogeneously with NaCl coated on the glass beads in the flow tube, resulting in the ion detection corresponding to HCl. Ozone was introduced into the system to inhibit the conversion reaction of Cl2 into HCl by removing H through the fast reaction of O3 + H. The mass spectrometric observation was that the O3 introduction reduced the concentration of HCl, while it increased the concentrations of Cl2 and ClO. The experimental results suggest that the nascent product of the titled reaction is gaseous Cl2, which is followed by fast conversion into HCl in presence of H. No significant difference in the concentration profile between under dry and wet (RH = 2%) conditions was observed. It is suggested that a further study including computational modeling will strengthen the evidence of the product identification.
![Anticorrelation between HCl ( ■ ) and Cl2 ( ○ ) as [H] increases (a) and [H] decreases in the presence of O3 (b).](http://oak.go.kr/repository/journal/12232/E1MPSV_2012_v3n4_108_f002.jpg)