The effect of cerium concentration and the addition of Sb2O3 on the light emission of cerium-contained glass were investigated. The glass matrix composition was 20Y2O3-25Al2O3-55SiO2, the CeO2 concentration ranged from 0.05 to 0.5 mol%, and Sb2O3 was added at concentrations of 0.02 to 0.1 mol%. The Ce3+ and Ce4+ absorption bands were observed at approximately 330 nm and 240 nm, respectively. A broad emission band at 400 nm, due to the 4f-5d transition of the Ce3+ ion, was observed under illumination by a UV light at 330 nm. The photoluminescence intensity of Ce3+ had a maximum value at a CeO2 concentration of 0.1 mol%. Adding Sb2O3 decreased the Ce4+ absorption intensity and enhanced the light emission intensity of Ce3+ by about 45%.
High power LEDs have undergone rapid development in recent years. The heat produced by high power LEDs induces deterioration of the transparent resin used for wrapping and fixing the powder phosphors onto the power chip [1]. As an alternative to resin, Tanabe et al. investigated the use of glass-ceramics for a novel durable phosphor for use in LED systems [1-2]. Glass phosphors for use in LEDs have also been investigated by several other research groups [3-5]. In spite of their good thermal durability, applying glass phosphors to LED products is quite difficult, because the light emission intensity and efficiency are relatively lower than those of the crystalline phosphors.
Glass does not have homogeneous site symmetry, therefore, the activator ion experiences a random distribution of local fields and, as a consequence, the light emission intensity is low [6-7]. Several activators also tend to build multivalent ion states in the glass [8-9]. In particular, cerium has two types of ions, viz. Ce3+ and Ce4+ [9]. However, only the Ce3+ ion has light emission properties. Therefore, if the Ce3+ proportion is increased in the glass, it is expected that the light emission efficiency of the glass phosphor could be enhanced, and potentially be applied to LED lamps.
The final goal of this study is to enhance the light emission intensity in the cerium-containing glass, by increasing the Ce3+ proportion. This study reports the effects of: 1) cerium concentration; and 2) addition of Sb2O3 as a reduction agent in cerium-containing 20Y2O3-25Al2O3-55SiO2 glass. To verify such effects, the light emission properties were characterized with various concentrations of CeO2 and Sb2O3.
20Y2O3-25Al2O3-55SiO2 glass samples were prepared using a conventional quenching method under oxidizing conditions. Commercial Y2O3, Al(OH)3 and SiO2 reagent grade powders were mixed by a 3D-turbulent mixer. The mixture was place in an alumina crucible and melted at 1550℃ for 4 h in an electric furnace. CeO2 was added to the host glass in the concentration range of 0.05 to 0.5 mol% and Sb2O3 was added from 0.02 to 0.1 mol%. The prepared glasses were annealed at the glass transition temperature, and thereafter, lapped and polished to 2 mm thickness and mirror surface. The light absorption spectra were measured by UV-visible (JASCO V-570) and the photoluminescence spectra were measured by PL-spectroscopy (PSI, DARSA Pro-5200, Xe light source) in the reflection mode.
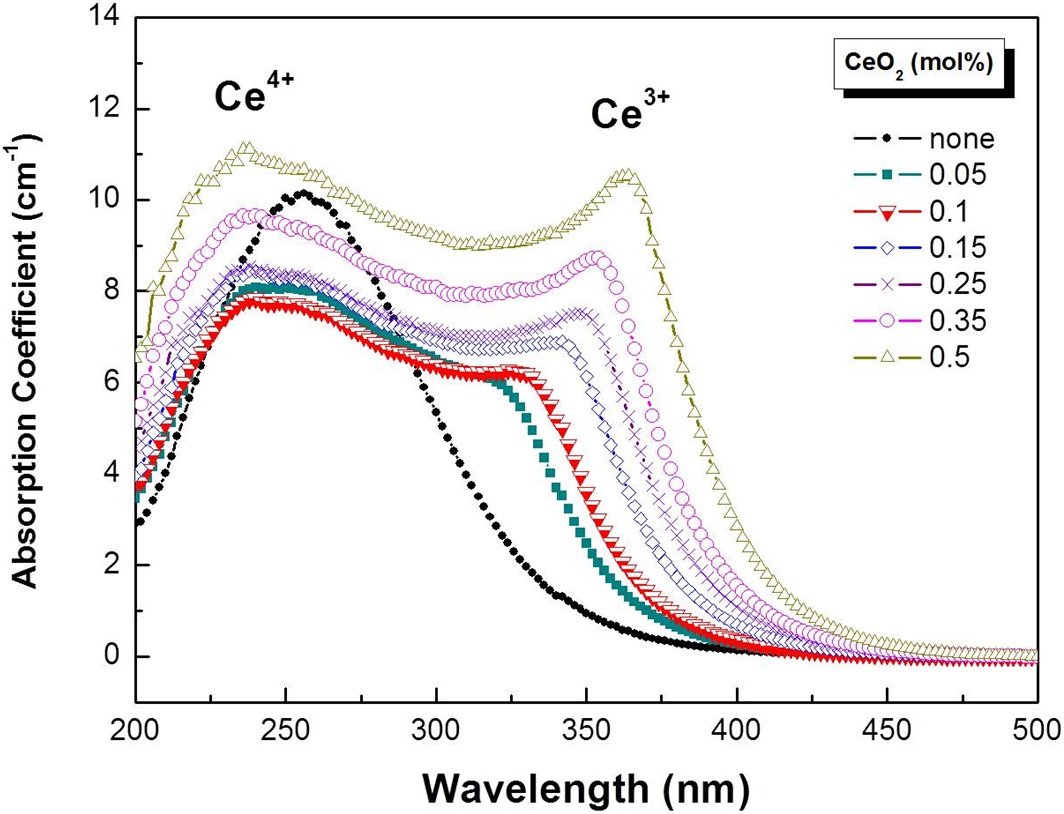
Figure 1 shows the optical absorption spectra of the cerium-containing 20Y2O3-25Al2O3-55SiO2 glasses. Two broad, strong absorption bands were observed in the UV region. Ce3+ exhibited an absorption band at around 330 nm and a broad absorption spectrum with a maximum value at 240 nm due to a charge transfer band of Ce4+.
The entire absorption intensity was increased and the Ce3+ absorption peak shifted to longer wavelengths as the cerium concentration increased.
Stroud reported a Ce3+ absorption band at 320 nm and a Ce4+ charge transfer band in the ultraviolet range in sodium-silicate glass [11]. Absorption spectra in this study showed good agreement with previous results (Fig. 1) [7,10].
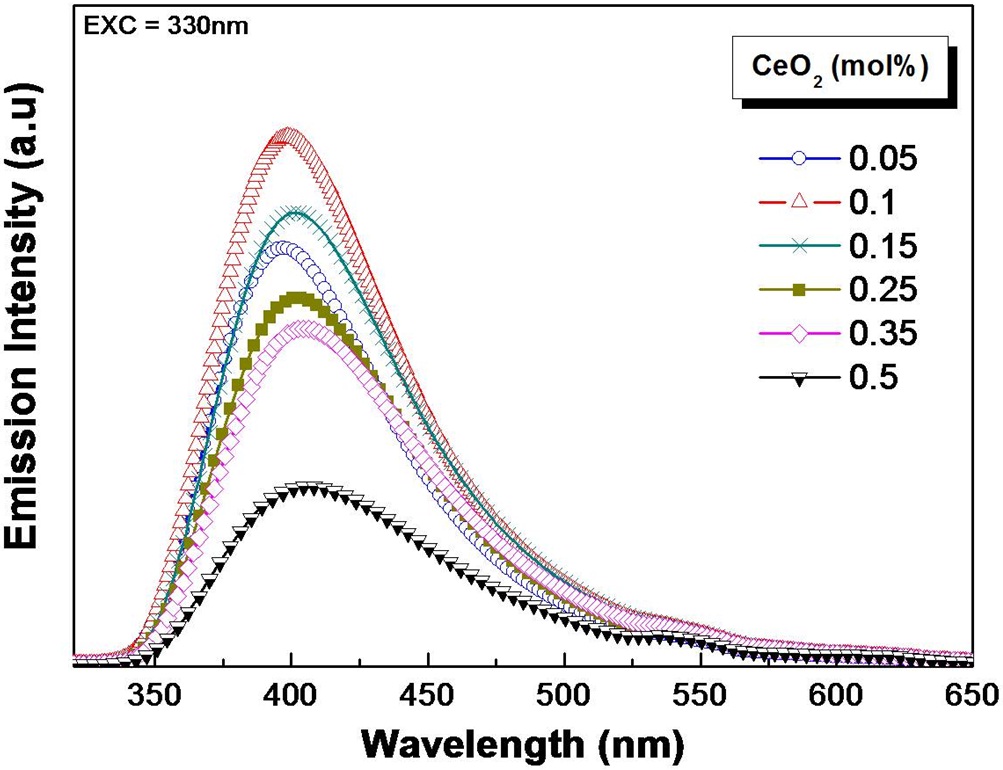
Figure 2 shows the Ce3+ emission spectra for different CeO2 amounts. There is a maximum value at approximately 400 nm upon 330 nm excitation. The emission intensity increased remarkably at a cerium concentration of 0.1 mol%. However, the emission intensity gradually decreased at cerium concentrations greater than 0.1 mol%. The decrease can be attributed to the concentration quenching due to the reduced ion-ion distance and the increased excited state interactions between Ce3+ ions.
Possible modification of the local structure due to increased CeO2 content may be responsible for the red-shift of the absorption and emission band of Ce3+ ions as the additional CeO2 can change the glass structure, producing non-bridging oxygen atoms and altering the covalency and optical basicity of nearby Ce3+ ions [14]. As covalency increases, the interaction between the electrons is reduced, so they spread out over wider orbitals [15]. Consequently, an increase in the covalency will reduce the energy difference between the ground and the excited states. This increase in covalency may lead to a shifting of the emission band and absorption band to a longer wavelength. A similar shift due to optical basicity has been observed in 6s-6p transition of Pb2+ [12]. The study has shown that the increase of optical basicity resulted from an increase of the degree of Pb-O covalency and led to the shifting of the absorption band to a longer wavelength. Thus, although the nephelauxetic effect [16] is a plausible cause of the red-shift, further study is required to investigate the changes within the local structure to fully understand the observed behavior.
3.2. The Effect of Cerium Ion Reduction by Sb2O3
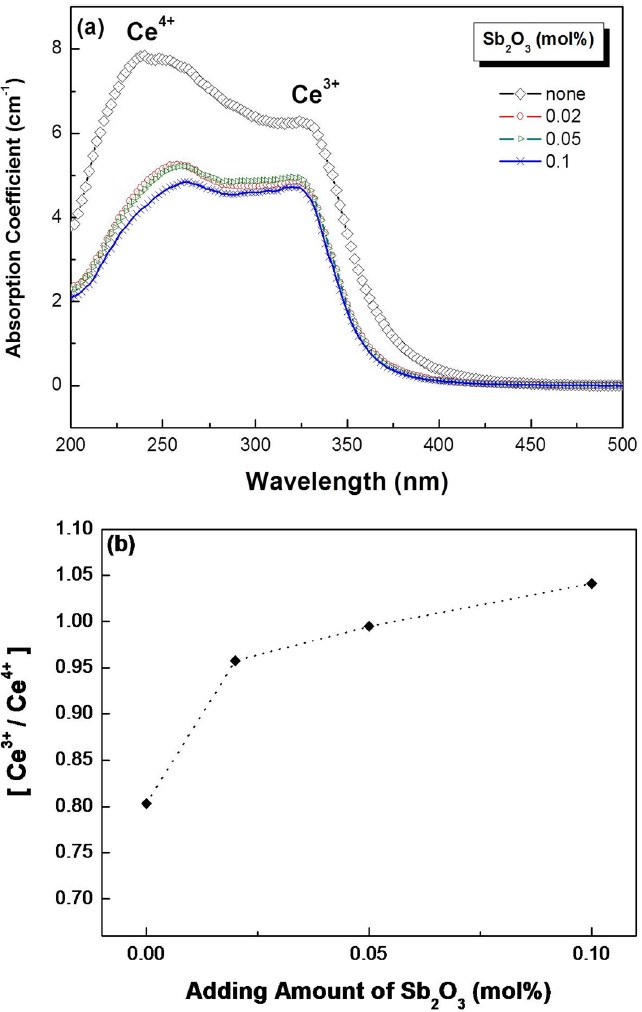
Figure 3(a) shows the optical absorption spectra for different amounts of Sb2O3 in 0.1 mol% CeO2 glass. The curves exhibited the typical spectra of Ce3+ ions and Ce4+ ions. The entire absorption intensity was radically decreased by introducing Sb2O3. A Ce3+ band shift due to changing the amount of Sb2O3 was not observed.
The addition of Sb2O3 has a proven reduction effect in the glass, similar to As2O3 [9]. Kim
The Ce3+ absorption spectra of Sb2O3-containing glasses
showed a similar line shape and distribution and there was no noticeable shift. The overall absorption intensity decreased upon introduction of Sb2O3. The reduced oscillation strength of the Ce-ions, which was induced by the local environmental change, may be responsible for the change. However, the origin of the overall decrease needs further study. It should be mentioned that the absorption peak due to Ce4+ decreased markedly compared to that of Ce3+. This clearly indicates the role of Sb2O3 reducing Ce4+ as suggested in equation (1).
Figure 3(b) indicates the value of the Ce3+/Ce4+ ratio for different Sb2O3 amounts. This ratio is simply expressed by the fraction of the Ce3+ peak value and the Ce4+ peak value. This calculation method suggested by Kim
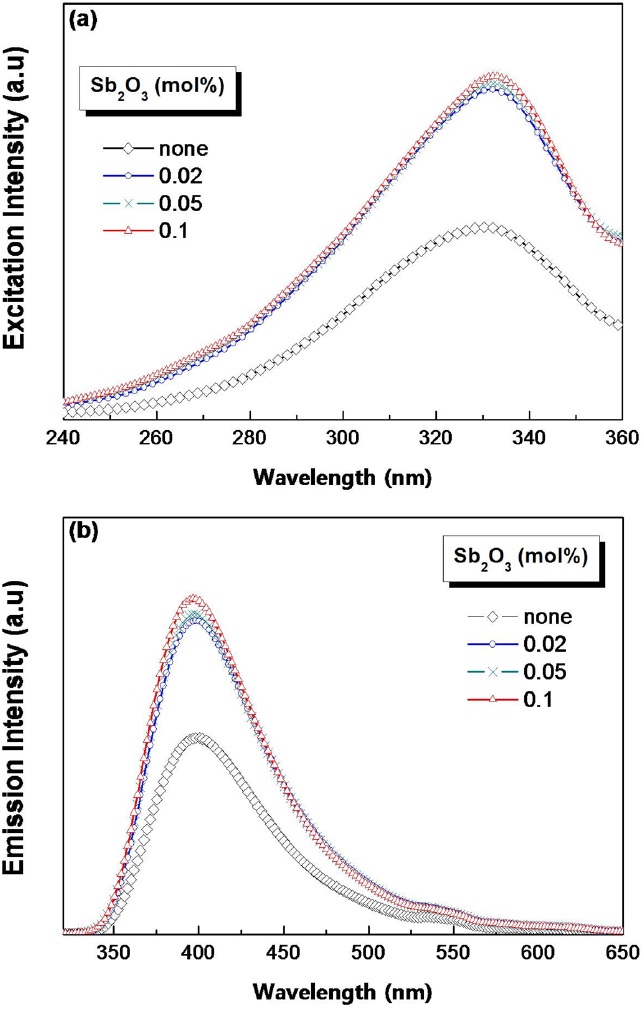
Figures 4(a) and (b) show the excitation and the
emission spectra, respectively, of Sb2O3-containing glasses. A strong excitation band was observed at 330 nm, regardless of the Sb2O3 amount. This value corresponded to the Ce3+ band of the absorption spectrum (Fig. 3(a)). The emission spectra exhibited the luminescence of Ce3+ ions with a maximum at 400 nm upon 330 nm excitation. Both the excitation and the emission intensity were enhanced by approximately 45 % compared to Sb-free glass. This enhancement is related to the increase of the Ce3+ activator concentration due to the Sb2O3 effect. As a result, the introduction of Sb2O3 to cerium-containing glass increased the proportion of Ce3+ ions and enhanced the intensity of the photoluminescence without atmospheric control.
Intrinsic light absorption and photoluminescence of ceriumcontained 20Y2O3-25Al2O3-55SiO2 glass were investigated. The resulting effect of cerium concentration and reduction with Sb2O3 was then studied.
The Ce3+ absorption band and emission band shifted to longer wavelengths according to an increase of the cerium oxide content due to the nephelauxetic effect. The light absorption intensity of the Ce4+ charge transfer band radically decreased as a result of the cerium ion reduction reaction with the introduction of Sb2O3. The proportion of Ce3+ was increased gradually and the ratio of Ce3+/Ce4+ increased linearly by increasing the amount of Sb2O3. Consequently, photoluminescence intensity was enhanced by approximately 45 % compared to that of Sb-free glass. Therefore, Sb2O3 is considered an effective reduction additive in the preparation of a glass phosphor that does not require atmospheric control.
This fundamental study is the basis for further investigations with regard to a YAG:Ce glass-ceramic phosphor.