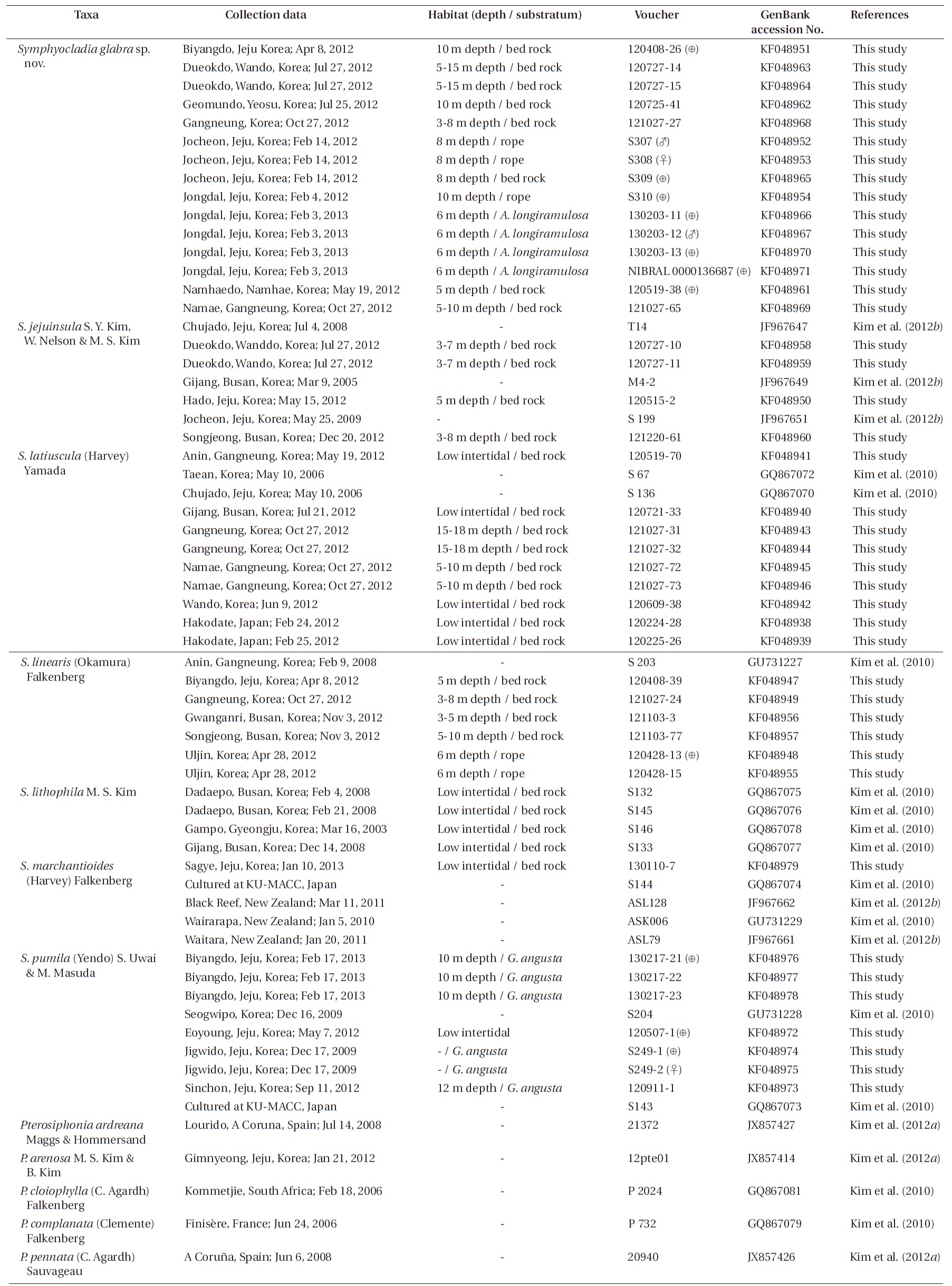
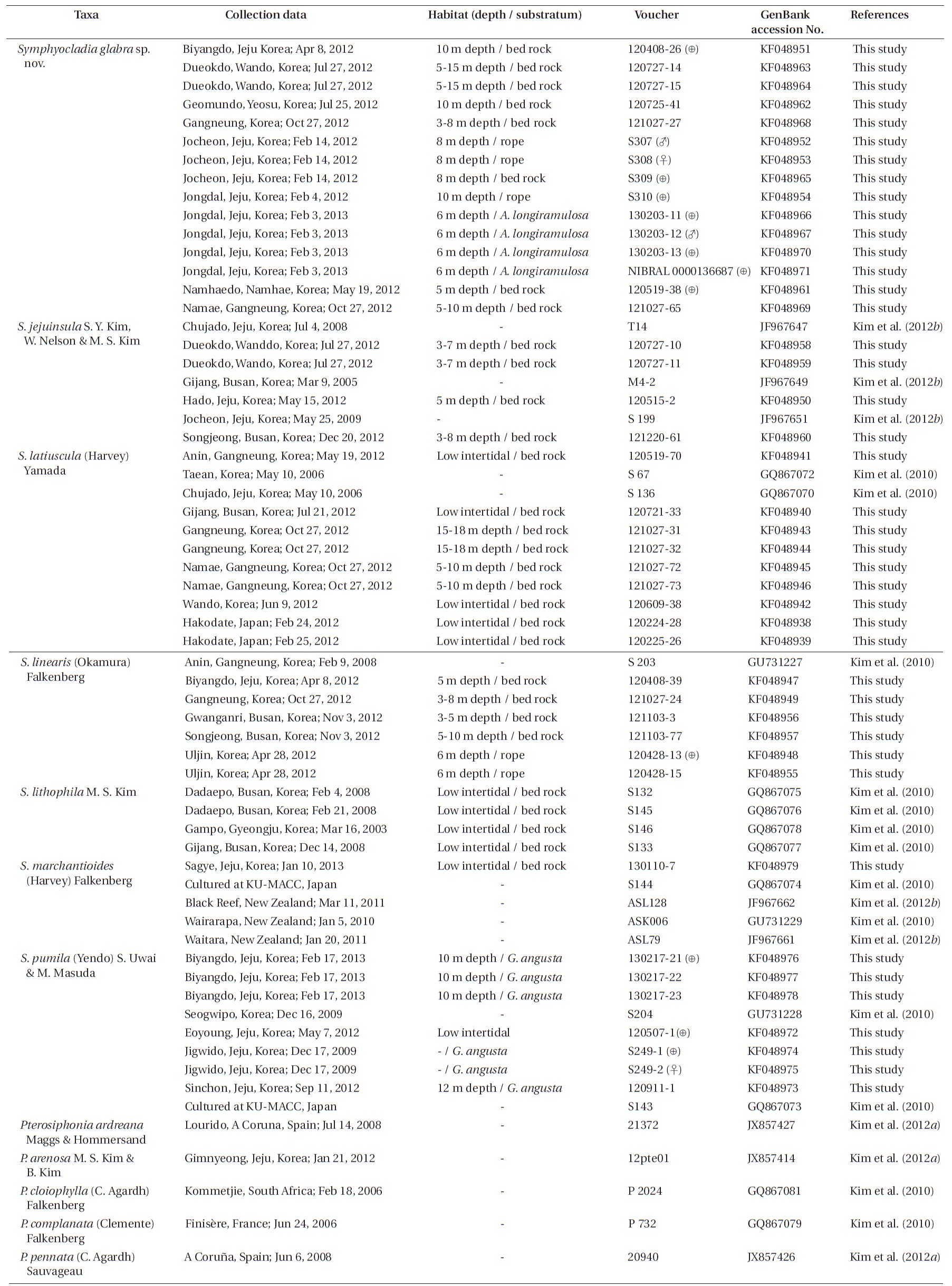
Six species of Symphyocladia are currently recognized including the type species S. marchantioides (Harvey) Falkenberg, and S. linearis (Okamura) Falkenberg, S. latiuscula (Harvey) Yamada, S. pumila (Yendo) Uwai and Masuda, S. lithophila M. S. Kim, and S. jejuinsula S. Y. Kim, W. Nelson and M. S. Kim. The genus Symphyocladia, a member of the Rhodomelaceae, was established by Falkenberg based on S. marchantioides, which was first reported by Harvey as Amansia marchantioides from New Zealand (Harvey 1855, Schmitz and Falkenberg 1897). Falkenberg (1901) established the tribe Pterosiphonieae and included Symphyocladia in this tribe. At this time, he transferred Placophora linearis Okamura (1895) (see De Toni 1895) from Japan and Dictyomenia gracilis Martens (1866) from China to the genus Symphyocladia. Yamada (1941) identified that S. gracilis (Martens) Falkenberg was the same species as Rytiphlaea latiuscula Harvey (1857) from Japan, and he made the combination S. latiuscula (Harvey) Yamada. Okamura (1923) added S. pennata from Japan; however, Uwai and Masuda (1999) reported that S. pennata is conspecific with Pterosiphonia pumila from Japan, which was first described by Yendo (1920) and they made the combination, S. pumila (Yendo) S. Uwai and M. Masuda. Recently, two more species (S. lithophila and S. jejuinsula) were added from the Korean coast (Kim et al. 2010, 2012b).
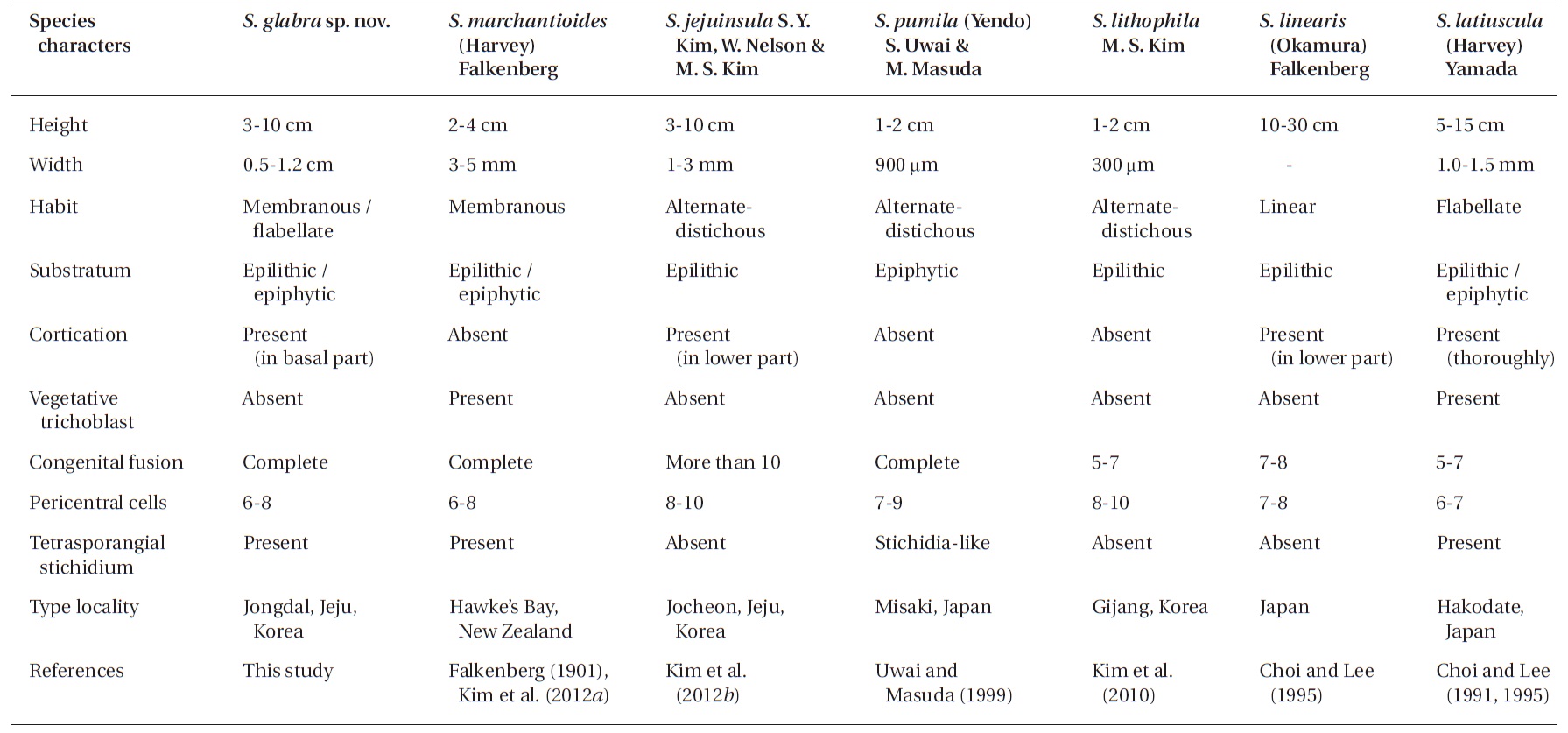
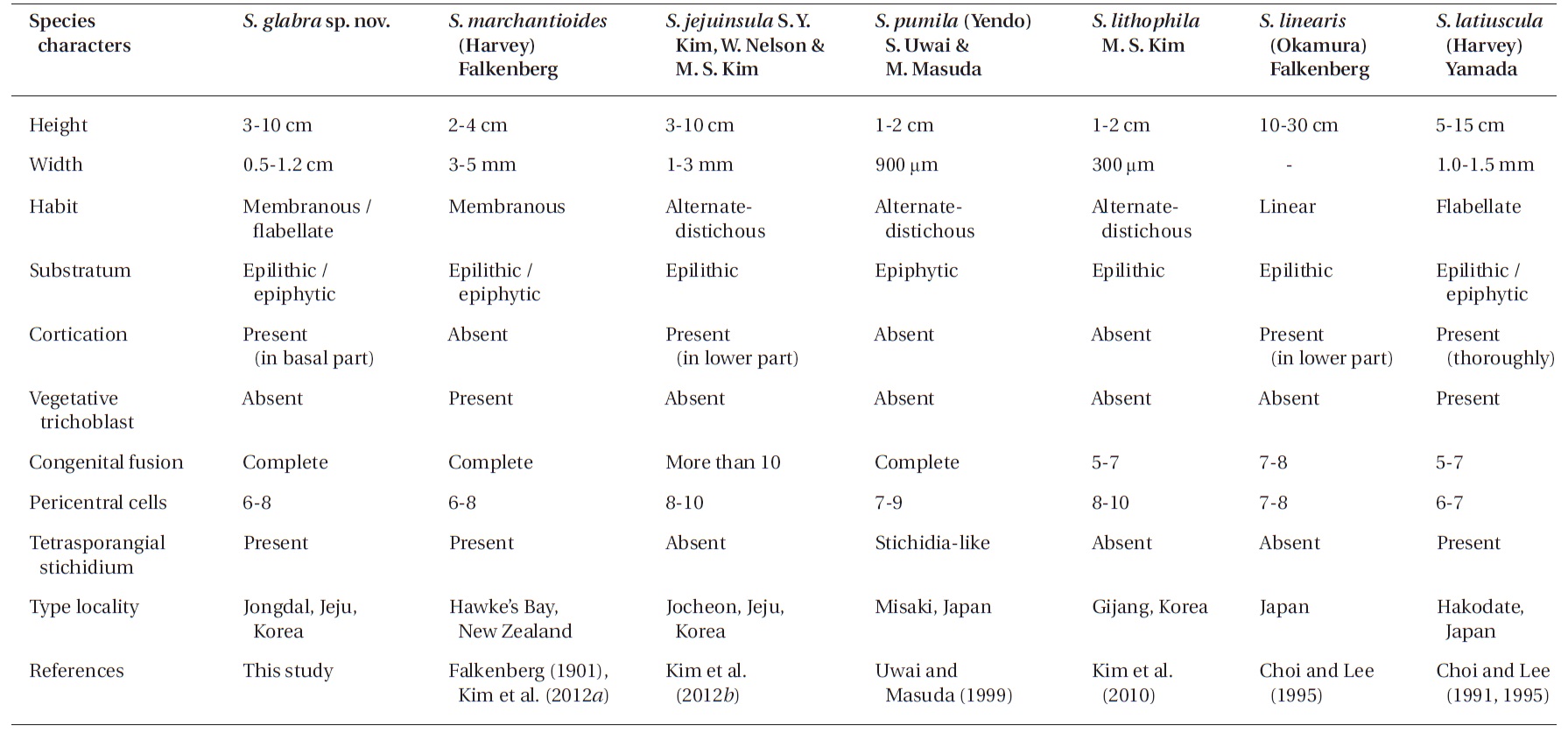
The morphological characteristics (e.g., habit of thallus, degree of cortication and congenital fusion, number of pericentral cells, occurrence of vegetative trichoblasts, and tetrasporangial stichidia) have been used to separate the symphyocladian species (Choi and Lee 1995, Uwai and Masuda 1999, Kim et al. 2010, 2012b). DNA sequences also have been widely used to determine the taxonomy and phylogeny of red algae, and they allow us to answer questions when identifying species without clear-cut morphological diagnostic criteria (Gavio and Fredericq 2002, Kim et al. 2004, 2012a, Conklin et al. 2009). In particular, the chloroplast-encoded rbcL gene is considered more phylogenetically informative in the genus or species level than the other markers for the red algae (Kim et al. 2010). For the symphyocladian species, rbcL sequences are the most useful marker in taxonomic study because the verified sequences through previous studies (Kim et al. 2010, 2012b) are available from GenBank.
It is reported that all of the symphyocladian species inhabit the Korean coast (Guiry and Guiry 2013). We collected species of Symphyocladia, including unidentified species, from several localities on the Korean coast. The unidentified specimens exhibited habits similar to those of S. marchantioides and S. jejuinsula in terms of thin, flat and flabellate thalli, and numerous apical cells in a roundish parallel shape. However, they were corticated on the lower part of thalli and had larger blades than S. marchantioides, and were not tapered upward in contrast with S. jejuinsula. Therefore, the aims of this study are as follows: 1) to determine whether the unidentified specimens are different species from S. marchantioides and S. jejuinsula; if so, 2) to confirm their taxonomic position in the genus Symphyocladia; and 3) to investigate the phylogenetic relationships based on the rbcL gene. To achieve these aims, we collected individuals of unidentified species in three phases of the life cycle including tetrasporophytes and male and female gametophytes, and analyzed their morphological and molecular characteristics.
Specimens were collected from the intertidal zone for S. latiuscula and S. marchantioides, and the subtidal zone for the other species by SCUBA. We used 40 symphyocladian samples from the Korean coast, two from Japan, and 21 rbcL sequence data of Symphyocladia and Pterosiphonia from previous studies. Field observations and collections of Symphyocladia sp. were made from eight sites on the eastern and southern coasts of Korea. The specimen collection data and the GenBank accession numbers of the rbcL data are listed in Table 1. Field-collected samples were put into an icebox with seawater and transported to the laboratory. Every individual was assigned an identification number, and photographs were taken with a D-80 camera (Nikon, Tokyo, Japan) on white paper. A part of the thalli was detached, cleaned in filtered seawater under the microscope to remove other organisms, and desiccated with silica-gel for DNA extraction, while the remaining major part of the thalli was used to make pressed specimens or preserved in 5% formalin / seawater for further morphological analysis. Sections were made using a freezing microtome (NK-101-II; Nippon Optical Works Co. Ltd., Tokyo, Japan). Sections on slides were stained with 1% aqueous aniline blue acidified with a drop of 1% HCl and mounted in 30% Karo corn syrup. Photomicrographs were taken using QIMAGING 1394 camera (QImaging, Surrey, BC, Canada) attached to a BX50 microscope (Olympus, Tokyo, Japan). Voucher specimens were deposited in the herbariums of Jeju National University (JNUB), Jeju and of National Institute of Biological Resources (NIBR), Incheon, Korea.
The total DNA of samples was extracted from the silica-gel dried specimens using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. The extracted DNA was stored at -20℃ and used to amplify the rbcL gene. Polymerase chain reaction (PCR) amplification was performed using rbcL primers designed by Kim et al. (2010). For samples the failed amplification, we redesigned the primers (e.g., rbcLJNF1 [5′-TGG TGA ATC ATC TAC AGC TA-3′] / rbcLJNR1 [5′-CAA TAC GCA TCC ATT TAC AA-3′] and rbcLJNF2 [5′-CAT AGA GCT GGT AAT TCA AC-3′] / rbcLJNR2 [TTC TAC AAA GTC AGC TGT ATC-3′] combination for the front and back part of the rbcL region, respectively). PCR amplification was performed in a total volume of 20 μL with AccuPower PCR PreMix (Bioneer, Daejeon, Korea). PCR was conducted with an initial denaturation at 95℃ for 5 min, followed by 35 cycles of amplification (denaturation at 94℃ for 1 min, annealing at 52℃ for 1 min, extension at 72℃ for 2 min), with a final extension at 72℃ for 7 min. The PCR products were purified using the AccuPrep PCR Purification Kit (Bioneer) and then sequenced commercially (Macrogen, Seoul, Korea). Both electropherogram outputs from each sample were edited using Chromas version 1.45 (Technelysium Pty Ltd., Helensvale, QLD, Australia). Total rbcL sequence was organized using the multiple-sequence editing program BioEdit (Hall 1999) and aligned visually. None of the alignments posed a problem, as no gaps were observed.
To confirm the taxonomic position of Symphyocladia sp., maximum likelihood (ML) analysis was performed using RAxML software (Stamatakis 2006) and the GTR + Г evolutionary model. Fifteen rbcL sequences of Symphyocladia
sp. were aligned with 43 of the other specimens of Symphyocladia, and five species of Pterosiphonia were used as an out group. We used 200 independent tree inferences using the -# option with default -I (automatically optimized subtree pruning and regrafting [SPR] rearrangement) and -c (25 distinct rate categories) options of the program to identify the best tree. To generate bootstrap values for the phylogenetic tree, we used the same program with the same settings for 1,000 replications.
Description. Thalli plerumque erecti, large plana et membranacea, rubro ad nigrantis purpurascentibus brunneis, attachiatus ad substratum, a pluribus singulae rhizoids a ventral pars implicati decumbentes palmes, rhizoids interibit de pricentral cellulis et terminatur in multicellulare haptera; erectis thalli 3-10 cm altus, 0.5- 1.2 cm in diameter et attenuatae ad basim, et latiores ad partem superiorem, divisa alternate vel opposite pinnatis modo, habere crenulate margine, corticated in basali pars deficimus midnervo lateralibus ramis omnino congenita traditio miscet parentali axe; numerosa apicalibus cellulis disposita surrotundus parallela figura; pericentral cellulis 6-8; vegetabilis trichoblasts rara, procarps sunt producatur in fugaces trichoblasts per laminae marginem cum quattuor-cellularibus carpogonial ramus, ostiolata cystocarp globosam cum polysiphonous culmus; spermatangia ortus in trichoblasts per margines superiores ense; antheridia adaxially curvus segeti figuram 1-2 monosiphonous culmus cellulis; tetrasporangiis sphaericum et tetrahedrally divisa, formatur unum per segmentum simplices ordines in lateraliter excoquuntur stichidia in marginibus laminis, 90 μm diameter, ab duabus circumventa lateralem et unum basali cover cellulis.
Thallus mostly erect, broadly flat and membranous, reddish to dark-purplish brown, attached to the substratum by numerous unicellular rhizoids from the ventral part of entangled decumbent branch, rhizoids cutting off from the pericentral cell and terminating in multicellular haptera; erect thallus growing 3-10 cm high, 0.5-1.2 cm in diameter and tapering at the base and wider toward the upper part, divided alternate or oppositely pinnate manner, crenulate margin, corticated in the basal part of the faint midrib; lateral branches completely congenital fused with the parental axis; numerous apical cells arranged in a roundish parallel shape; pericentral cells 6-8; rare vegetative trichoblasts; procarps producing on fugacious trichoblast along the margins of the blade with a four-celled carpogonial branch; ostiolate globular cystocarp with a polysiphonous stalk; spermatangia bearing on trichoblast along the upper margins of the blade; corn-shaped antheridia adaxially curved with 1-2 monosiphonous stalk cells; tetrasporangia spherical and tetrahedrally divided, formed one per segment in straight rows in laterally fused stichidia on the margins of the blade, surrounded by two lateral and one basal cover cell.
Holotype. JN130203-11 (tetrasporophyte), collected from 6 m depth of Jongdal, Jeju province, Korea (33°29′ 53′′ N, 126°54′50′′ E) on Feb 3, 2013, and deposited in the JNUB (the Herbarium of Department of Biology, Jeju National University, Korea).
Isotypes. JNUB (JN130203-12-13) and KB (NIBRAL00 00136687-8).
Distributions. Collected from Jeju Island, and the southern (Wando to Namhaedo) and eastern (Gangneung) coasts of Korea.
Etymology. The specific epithet (glabra) was chosen to represent the morphological difference from the type species, S. marchantioides, as lack of hair.
Korean name. 민털보라색우무.
Habitat. Symphyocladia glabra was collected at 3-30 m depth of the subtidal zone and they were growing on bed rock, abandoned rope or other red algae, such as Acanthopeltis longiramulosa, Gelidium elegans. Reproductive fronds were mostly discovered between winter and spring season.
Other specimens examined. Jocheon, Jeju (JN 110520, tetrasporophyte, May 20, 2011; S 307, male gametophyte, Feb 14, 2012; S 308, female gametophyte, Feb 14, 2012; S309, tetrasporophyte, Feb 14, 2012); Gwideok, Jeju (JN110531, tetrasporophyte, May 31, 2011); Udo, Jeju (JN130203-7, female gametophyte, Feb 3, 2013); Jongdal, Jeju (S310, tetrasporophyte, Feb 4, 2012); Biyangdo, Jeju (JN120408-26, tetrasporophyte, Apr 8, 2012); Namhaedo, Namhae (JN120519-38, tetrasporophyte, May 19, 2012); Geomundo, Yeosu (JN120725-41, vegetative thallus, Jul 25, 2012); Dueokdo, Wando (JN120727-14-15, vegetative thallus, Jul 25, 2012); Gyeongpo, Gangneung (JN121027- 27, vegetative thallus, Oct 27, 2012); Namae, Gangneung (JN121027-65, vegetative thallus, Oct 27, 2012).
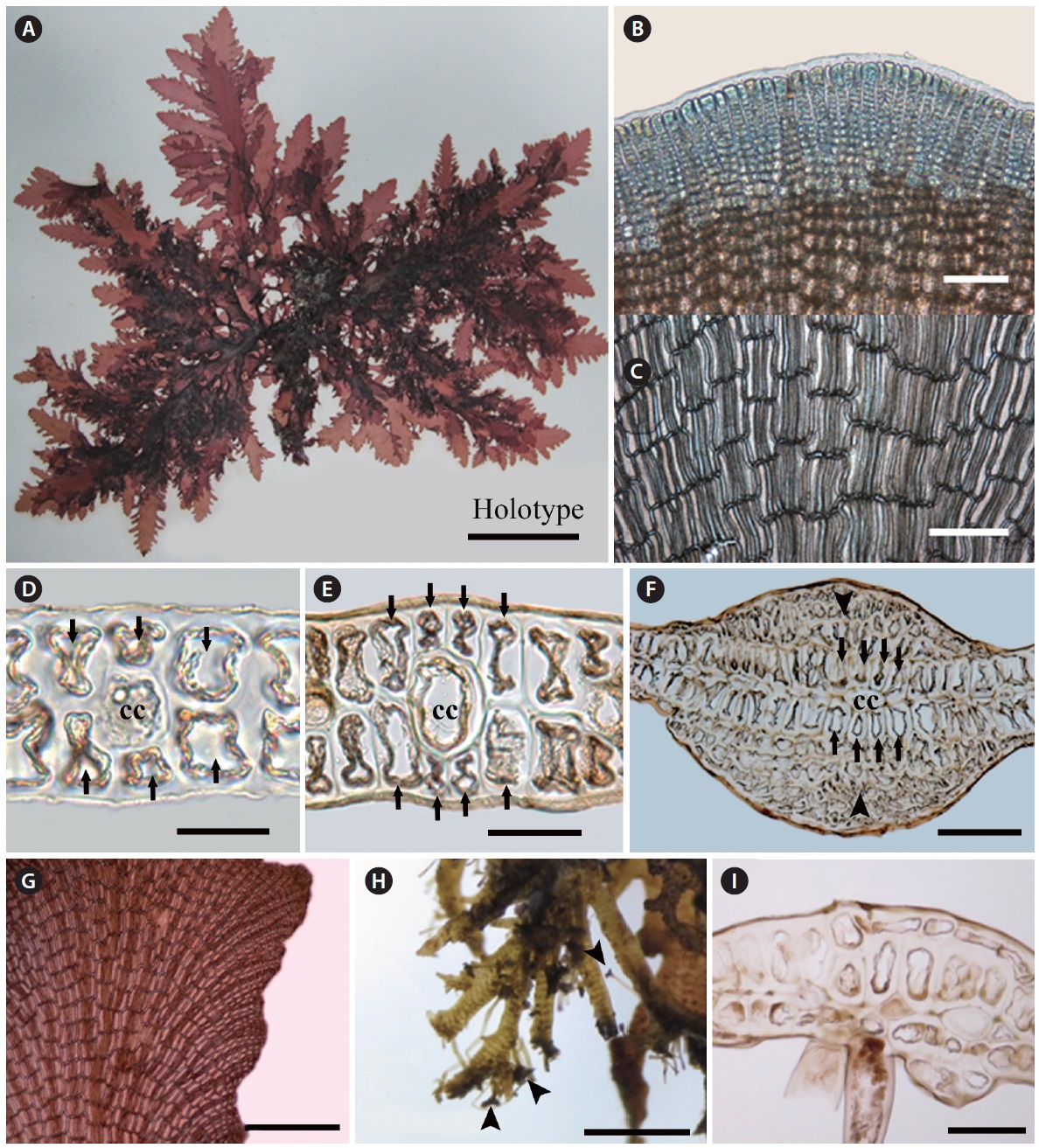
Morphology. Fronds are mostly erect. Erect thalli are thin, flat, and broadly membranous; 3-10 cm high; 0.5-1.2 cm wide; and 200 μm thin in the middle part of the blade, tapering at the base and wider toward the upper part (Fig. 1A). Growth occurs from numerous apical cells, which are
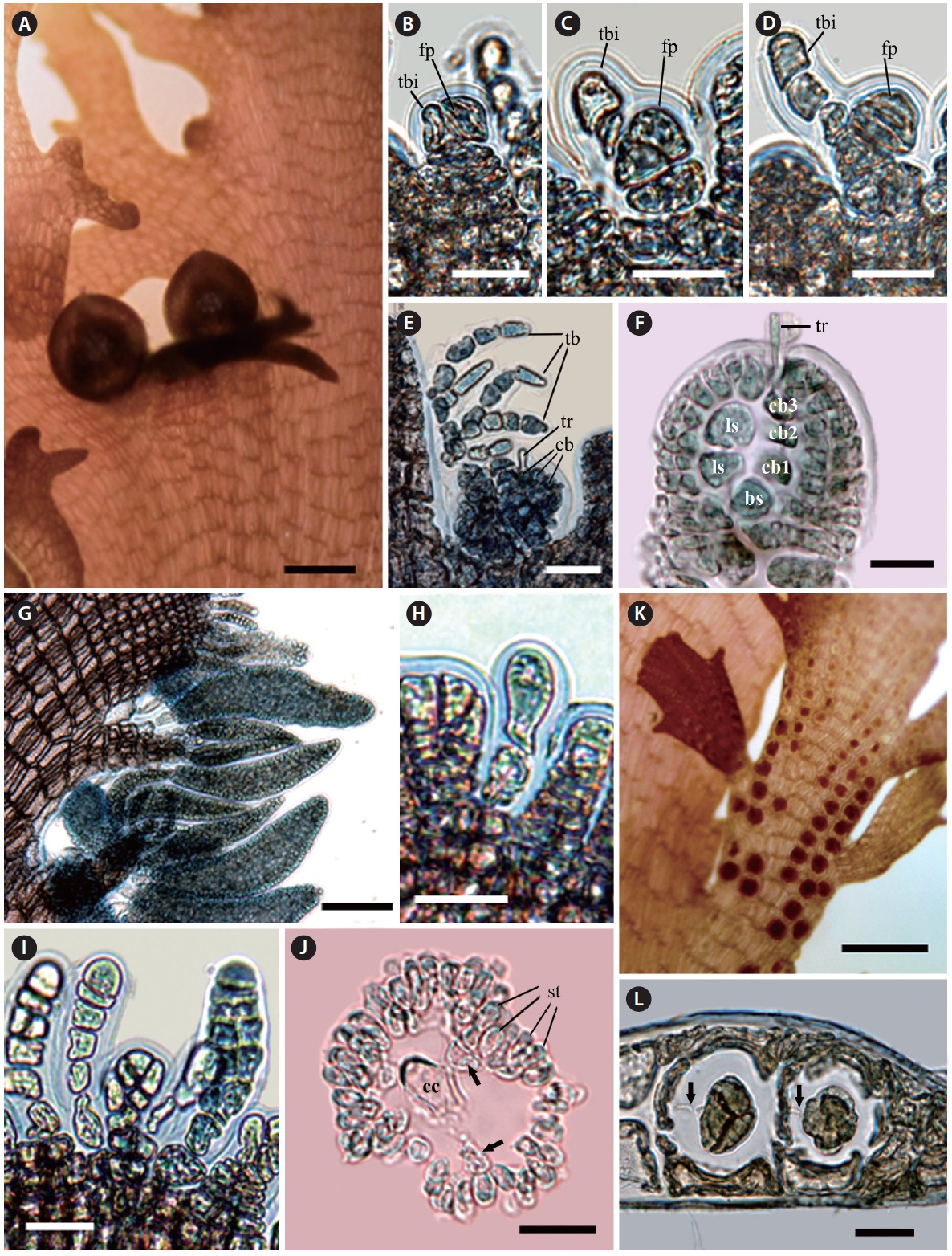
[Fig. 1.] Vegetative structure of Symphyocladia glabra sp. nov. (A) Holotype specimen from Jongdal (JN130203-11), Jeju Island. (B) Apical cells showing roundish parallel arrangement. (C) Surface view of middle part of thallus showing congenitally fused polysiphonous filaments. (D-F) Cross section views of the parts of thallus showing a central cell (cc) surrounded by 6-8 pericentral cells (arrows), and cortication (arrowheads) on the basal part, the below apical (D), the middle (E), and the basal part (F). (G) Thallus margin showing undulate shape and lack of vegetative trichoblast. (H) Decumbent branches attached by numerous unicellular rhizoides forming haptera (arrowheads). (I) Rhizoid cutting off from the pericentral cell of decumbent branch. Scale bars represent: A, 3 cm; B & E, 50 μm; C & H, 100 μm; D, 1,000 μm; F, 500 μm; G, 30 μm; I, 300 μm.
arranged in a roundish parallel shape. The apical cells are divided obliquely at first, cutting off one segment transversely downward and forming pericentral cells in an alternate sequence. The apical cells and pericentral cells of the thalli become congenitally fused with the row of axial cells (Fig. 1B). In the surface view, mature pericentral cells are arranged in a nodular fan shape, about 30-40 μm in diameter and 200-300 μm long (Fig. 1C). In the transverse section, the single central cell is surrounded by six to eight pericentral cells (Fig. 1D & E). The main branch has a faint midrib, which is corticated in the basal part (Fig. 1F). Branches are divided alternate or oppositely in a pinnate manner in a plane and become a crenulate margin (Fig. 1A & G). The erect thalli are occurred from the entangled decumbent branch, which adheres to the substratum by numerous protruding rhizoids from the under-surface (Fig. 1H). Rhizoids are unicellular, 30 μm in diameter, cutting off from the pericentral cell of the decumbent branch and forming multicellular haptera on their terminal part (Fig. 1H & I). No vegetative trichoblasts are observed. The color is bright red when alive but becomes light brown when fixed by formalin solution. The texture is membranous and adheres well on a paper.
The procarps are initiated with forming trichoblast initial and the fertile pericentral cell derived from the apical cell. The apical cell cuts off the fertile pericentral cell obliquely and becomes the trichoblast initial itself. The trichoblast initial is divided transversely and is alternately branched three times, and it consequently forms a reproductive trichoblast that is bent adaxially (Fig. 2B-E). At the same time, the fertile pericentral cell is more developed, and it forms the basal sterile initial and the four-celled carpogonial branch (Fig. 2E & F). The procarps are covered prior to fertilization by a pericarp derived from the periaxial cells of the suprabasal reproductive trichoblasts (Fig. 2E & F). Before maturation of the carpogonium, the reproductive trichoblasts are decayed. The mature ostiolate cystocarps are globular, 400-500 μm in diameter and have polysiphonous stalks (Fig. 2A).
Spermatangial branchlets are produced on the protuberances along the upper margins of the blades (Fig. 2G). The spermatangial branchlets are initiated with the modification of apical cells to unbranched and monosiphonous trichoblasts by several transverse cell divisions. The monosiphonous trichoblasts are born spirally in an alternate manner on every segment of the marginal protuberance, and they become polysiphonous by longitudinal division (except basal 1-2 cells). The mature spermatangial branchlets are corn-shaped, 300-350 μm long, 70-90 μm in diameter, and curved adaxially (Fig. 2G-J).
The tetrasporangia are spherical, divided tetrahedrally, and produced one per segment in straight rows on the laterally fused stichidia at the margins of the blades. The fertile branchlets swell on both sides with tetrasporangial maturity. The mature tetrasporangia are 90 μm in diameter with two cover cells. The tetrasporangia are connected with a stalk cell by a pit-connection (Fig. 2K & L). No vegetative trichoblasts are observed.
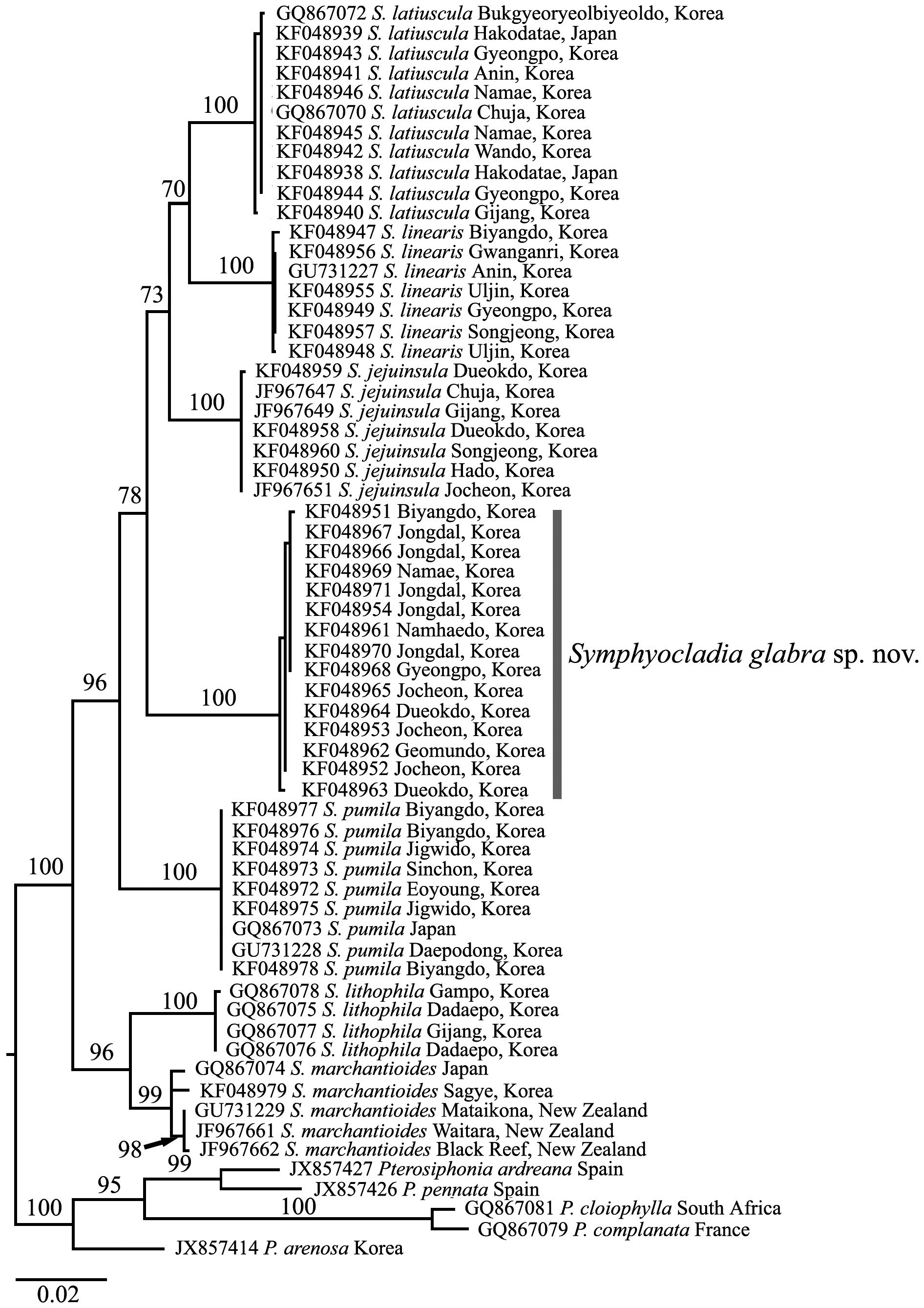
Molecular analysis. We aligned 1,150 nucleotide base pairs of the rbcL gene from 58 individuals of symphyocladian specimens including 15 S. glabra, and five pterosiphonian species as an outgroup. Of all sites, 208 (18.1%) were variable and 176 (15.3%) were phylogenetically informative. The 15 specimens of S. glabra from eight sites in Korea formed an independent monophyletic clade with 0-0.3% divergence that separated from the other clades. There was a similar divergence between S. glabra and the other Symphyocladia species by 4.3-4.8%, except S. lithophila by 5.2-5.4%, which had the highest difference among the Symphyocladia clades.
In the phylogenetic tree (Fig. 3), the genus Symphyocladia was clearly separated from the Pterosiphonia clade and supported monophyly by the 100% ML bootstrap value. Each species of Symphyocladia including S. glabra, also formed independent clades with strong bootstrap values.
We intensively collected symphyocladian species along the Korean coast and found a new species named Symphyocladia glabra sp. nov. This species is morphologically similar to S. marchantioides in characters of the flabellate and pinnate outline, the faint midrib and roundish crenulate margin of the blade, the complete congenital fusion of the axis with laterals, the roundish parallel arrangement of the numerous apical cells, the number of pericentral cells, and the production of tetrasporangial stichidia. However, they differ in thallus size, the lack of vegetative trichoblasts, and the cortication on the lower portion of the midrib (Kim et al. 2012b). Moreover, S. glabra also closely resembles to S. jejuinsula having the features of the mostly erect thalli, the membranous flabellate erect branches, the cortication on the basal part of the thalli, and the lack of vegetative trichoblasts. Conversely, S. jejuinsula has more slender and tapering branches toward the apical part, 10 segments of congenitally fused branchlets, and eight to ten pericentral cells (Kim et al. 2012b). While comparing new species with the other species of Symphyocladia, we found the obvious morphological characters separating them from the others (Table 2). The development of reproductive structures is essentially the same as in other symphyocladian species, such as S. pumila (Uwai and Masuda 1999), and S. latiuscula (Matsuyama and Masaki 1975, Choi and Lee 1991). The recognition of this new species is strongly supported by not only morphological but also molecular evidence. Kim et al. (2012b) mentioned that the divergence of the rbcL sequence among the species of Symphyocladia ranges
[Fig. 2.] Reproductive structure of Symphyocladia glabra sp. nov. (A) Female reproductive organ producing on the upper margin of blade. (B-F) Development of female reproductive organ (bs, basal sterile cell; cb, carpogonial branch; fp, fertile pericentral cell; ls, lateral sterile cell; tb, trichoblast; tbi, trichoblast initial; tr, tricogyne). (G) Spermatangial branchlets producing along the upper margin of blade. (H & I) Development of male reproductive organ. (J) Cross-section view of mature spermatangial branchlet showing a central cell (cc) and pericentral cells (arrows) surrounded by numerous spermatangia (st). (K) Tetrasporangial stichidia producing on the upper margin of blade. (L) Development of tetrasporangia, which are connected with a stalk cell by a pit-connection (arrows). Scale bars represent: A, 500 μm; B-D, H-J & L, 20 μm; E & F, 50 μm; G, 100 μm; K, 300 μm.
between 2.6-5.6%. From rbcL data in this study, the new species had more than 4% of divergence and formed a monophyletic clade strongly supported by the 100% ML bootstrap value. The results of our molecular analysis suggest that the chloroplast-encoded rbcL gene is very useful marker in delimitating morphologically similar taxonomic groups in the genus Symphyocladia.
It is possible that in the Northwestern Pacific region, three similar symphyocladian species (i.e., S. marchantioides, S. jejuinsula, and S. glabra) have been identified to one species as S. marchantioides. Okamura (1912) illustrated four specimens of S. marchantioides, one large and three small. The largest specimen has morphological features of the many linear prostrate branches and the erect thallus tapering upward that are similar to those of S. jejuinsula. His description is also consistent with the description of S. jejuinsula by Kim et al. (2012b) in feature of thallus habit and size, and cortication of midrib.
In Korea, Lee (2008) described S. marchantioides in his pictorial book on macroalgae. We observed the specimens identified as S. marchantioides (Lee 2008) deposited in the JNUB, which were voucher specimens of the plate on page 321. As a result, we confirmed that the two specimens collected from Biyangdo (sample No. 2006- 138) and Jeju Harbor (sample No. 2007-72) had the same characteristics as S. glabra in terms of thallus habit, lack of vegetative trichoblasts, thallus size, and cortication on the lower portion of the faint midrib.
In the genus Symphyocladia, the following diagnostic characters for classification at the species level have been used: habit of thallus, degree of cortication and congenital fusion, number of pericentral cells, and occurrence of vegetative trichoblasts and tetrasporangial stichidia (Choi and Lee 1995, Kim et al. 2010, 2012b). The other four species are easily distinguishable by their morphological characteristics from S. marchantioides, S. jejuinsula, and S. glabra as follows: 1) S. linearis by its linear and large thalli, seven to eight pericentral cells, and proximal cortication; 2) S. latiuscula by its thick and entirely corticated thalli (except on the apical part of the blade), abundant vegetative trichoblasts, and six to seven pericentral cells; 3) S. pumila by its small ecorticate thalli with alternate-distichous branches at two-segment intervals, seven to nine pericentral cells, seven to eleven segments congenitally fused between lateral branches and parental axes, and stichidia-like tetrasporangial branchlets; and 4) S. lithophila by its small ecorticated thalli with alternatedistichous branches on every second segment of the axes, eight to ten pericentral cells, congenital fusions in five to seven segments, and epilithic habitation (Table 2).
In conclusion, we intensively collected symphyocladian species along the Korean coast and revealed (after adding a new species) that, in total, seven species inhabit in the Korean coast. Based on morphological and molecular analysis, we identified one novel species, Symphyocladia glabra sp. nov. that has been known as S. marchantioides. Therefore, three morphologically similar Symphyocladia species are co-existing in the Northwestern Pacific region (i.e., S. glabra, S. marchantioides, and S. jejuinsula). In addition, it is very interesting that S. marchantioides exists in both the northern and southern hemisphere (Fig. 3). The specimens of S. marchantioides from Korea and New Zealand showed genetic variation of 0.6-0.7%. To elucidate the distribution of S. marchantioides in both hemispheres, we need more evidence from various geographical regions.