The genus Haematococcus (Chlorophyceae, Volvocales) is cosmopolitan, reported from all continents except Antarctica (Guiry and Guiry 2013). Seven species of Haematococcus have been currently accepted taxonomically and among them, H. pluvialis Flotow is the best studied. This ubiquitous microalga occurs primarily in principally ephemeral small fresh water pools in temperate climate, where it passes through the life cycle in as few as 4 days (e.g., Droop 1954, Czygan 1970, Thompson and Wujek 1989). It is well known for the ability to synthesize and accumulate esters of the red ketocarotenoid astaxanthin (3,39-dihydroxy-b,b-carotene-4,49-dione) for up to 4% of the total cellular dry weight under laboratoryinduced stress conditions, such as nutrient deprivation, increased salinity, high irradiance, and exposure to high temperature (30-33℃), as well as combinations of these stresses (summarized in Collins et al. 2011). In natural environment, accumulation of the astaxanthin is an adaptation to habitats that exhibit strong radiation, in addition to the formation of cysts having rigid cell walls (e.g., Hagen et al. 1994, 2002, Montsant et al. 2001).
Astaxanthin is generally known to be used as a food coloring agent, natural feed additive for the poultry industry and for aquaculture, especially as a feed supplement in the culture of salmon, trout and shrimp, and for medicinal and cosmetic application due to its powerful antioxidant capacity. Most of the astaxanthin used for aquaculture is synthetically derived; however, growing demand exists for commercial production of astaxanthin from natural sources. Therefore, numerous studies regarding Haematococcus focused on the astaxanthin-producing properties of H. pluvialis, using strains mainly from culture collections (i.e., The Culture Collection of Algae at the University of Texas at Austin [UTEX], National Institute for Environmental Studies, Japan [NIES], Culture Collection of Algae and Protozoa [CCAP], The Global Bioresource Center [ATCC], Culture Collection of Algae at Goettingen [SAG], Algae Culture Collection at Norsk Institutt for Vannforskning [NIVA]) and grown under a wide range of culture conditions (Gonzalez et al. 2009). The usual growth temperature for H. pluvialis is between 20-27℃.
We recently found a new cold-adapted Arctic strain of Haematococcus on Blomstrandhalvøya Island (Svalbard) (Kim et al. 2011). Our strain, tentatively named as Haematococcus sp. (Kim et al. 2011), exhibits growth between 4-15℃, whereas the average summer temperature of Svalbard reaches 4-6℃, and January averages at -12℃ to -16℃. The purpose of the present study was to provide more morphological and physiological details for this peculiar Arctic strain of Haematococcus and to address its phylogenetic relationships to other species in the genus.
Specimen of Haematococcus pluvialis was collected on Blomstrandhalvøya Island (78°59′ N, 12°03′ E) in a small freshwater basin in the rock fed with melted snow by the authors G. H. K., T. A. K., and J. W. H. (Kim et al. 2011). The unialgal strain was isolated as described by Kim et al. (2011) and grown in a modified liquid ATCC Medium 625 (Klochkova et al. 2006), Bold’s basal medium (Bischoff and Bold 1963), and conventional BG11 medium in 90 × 60-mm plastic Petri dishes and 150-mL glass flasks at 4-15℃, 30-35 μmol photons m-2 s-1 provided by cool-white fluorescent lighting and 12 : 12 h light : dark regime. Micrographs were taken with Olympus DP50 digital camera (Olympus, Tokyo, Japan) affixed to an Olympus BX50 microscope using Viewfinder Lite and Studio Lite computer programs.
For investigation of chloroplast morphology, cells were observed under an Olympus Fluoview laser scanning confocal microscope. The microscope was equipped with an argon-krypton laser using a 488-nm excitation line and AOBS filter-free system collecting emitted light between 498 and 700 nm. A series of optical sections of chloroplast was captured and used for 3D reconstruction of morphology. The autofluorescence of the chlorophyll was exploited for visualization of chloroplast structure.
Three fixation methods were applied to fix cells in vegetative (green) and aplanospore (red) stages, including chemical fixation, cryofixation, and high-pressure freezing. The chemical fixation and embedding methods were performed as described by Klochkova et al. (2006), and the cryofixation and embedding methods were performed as described by Terauchi et al. (2012). For high pressure freezing and freeze substitution, a pellet of cells was placed between two copper planchets and rapidly frozen in a high-pressure freezing instrument (EM PACT2; Leica, Solms, Germany) at a pressure of approximately 2,000 atm and at the temperature of liquid nitrogen. The samples were then placed in liquid nitrogen, and the top of the copper tube was peeled away for further processing. The following cryofixation and embedding methods were performed as described by Terauchi et al. (2012). Resin-embedded samples were sectioned with a diamond knife, and thin sections stained with 4% uranyl acetate for 30 min and lead citrate (Reynolds 1963) for 10 min were viewed and photographed on a Hitachi H-300 transmission electron microscope (TEM) (Hitachi, Tokyo, Japan).
The DNA of H. pluvialis was extracted using an Invisorb Spin Plant Mini Kit (Invitek, Berlin-Buch, Germany) according to the manufacturer’s protocol. Extracted DNA was stored at -20℃ and used for the amplification of 18S rRNA. The 18S rRNA was amplified and sequenced using the following primers: G01 (5′-CACCTGGTTGATCCTGCCAG-3′), G14 (5′-CCTTGGCAGACGCTTTCGCAG-3′), G04 (5′-CAGAGGTGAAATTCTTGGAT-3′), and G07 (5′-GCTTGATCCTTCTGCAGGTTCACCTAC-3′) (Saunders et al. 1995). Polymerase chain reaction and sequencing were performed as detailed in Klochkova et al. (2006, 2008). All sequences of the forward and reverse strands were determined and the electropherograms were edited using the program Chromas v.1.45 (McCarthy 1998). Nucleotide sequences were aligned using Se-Al v2.0a11 (Rambaut 2002).
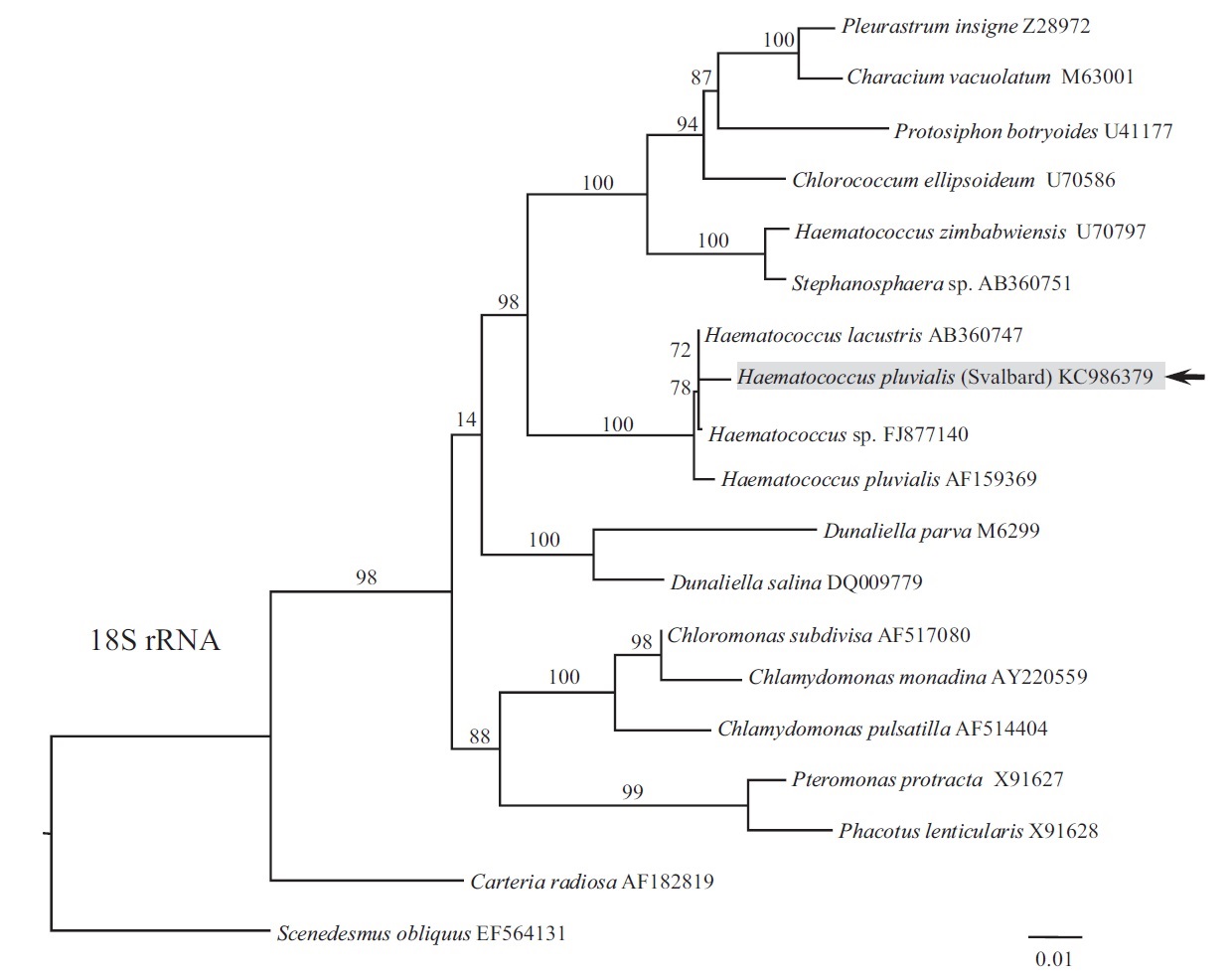
Phylogeny of 18S rRNA was reconstructed using maximum likelihood (ML). ML analyses were performed with RAxML v.7.2.8 (Stamatakis 2006) using the GTRGAMMA model. We used 300 independent tree inferences, applying options of automatically optimized surface plasmon resonance (SPR) rearrangement and 25 distinct rate categories in the program to identify the best tree. Statistical support for each branch was obtained by 1,000 bootstrap replications with the same substitution model. The sequence determined in this study has been deposited in GenBank under accession number KC986379.
Our strain nested within the Haematococcus group (Fig. 1), forming a sister relationship to H. lacustris (AB360747; isolate NIES-144, collected from Sapporo, Hokkaido) and Haematococcus sp. (FJ877140; origin unknown). The clade included H. pluvialis (AF159369; isolate SAG 34-1b, collected from former Czechoslovakia), although it had less affinity to our strain. Species H. lacustris has been synonymized with H. pluvialis. If they are conspecific, our strain positioning between them should also be attributed to H. pluvialis until more information is available on various strains of the H. pluvialis-lacustris complex.
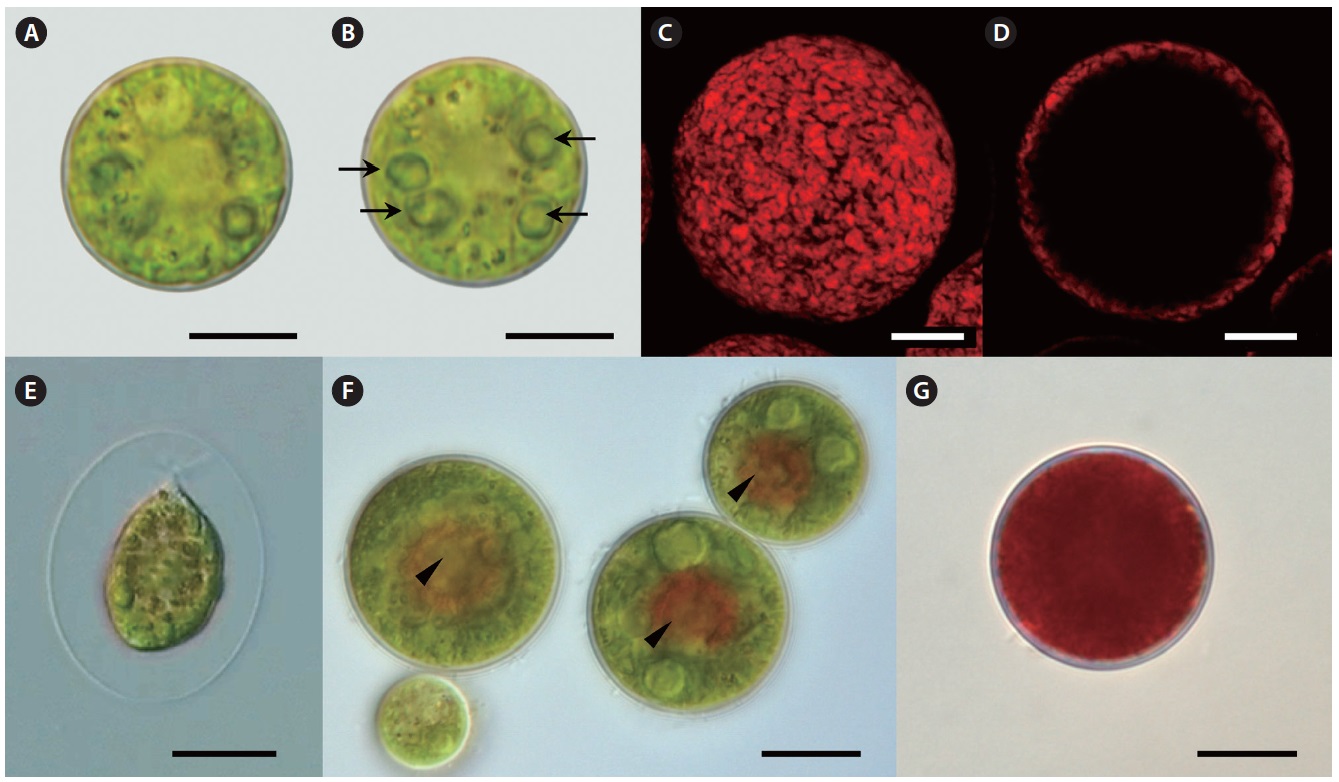
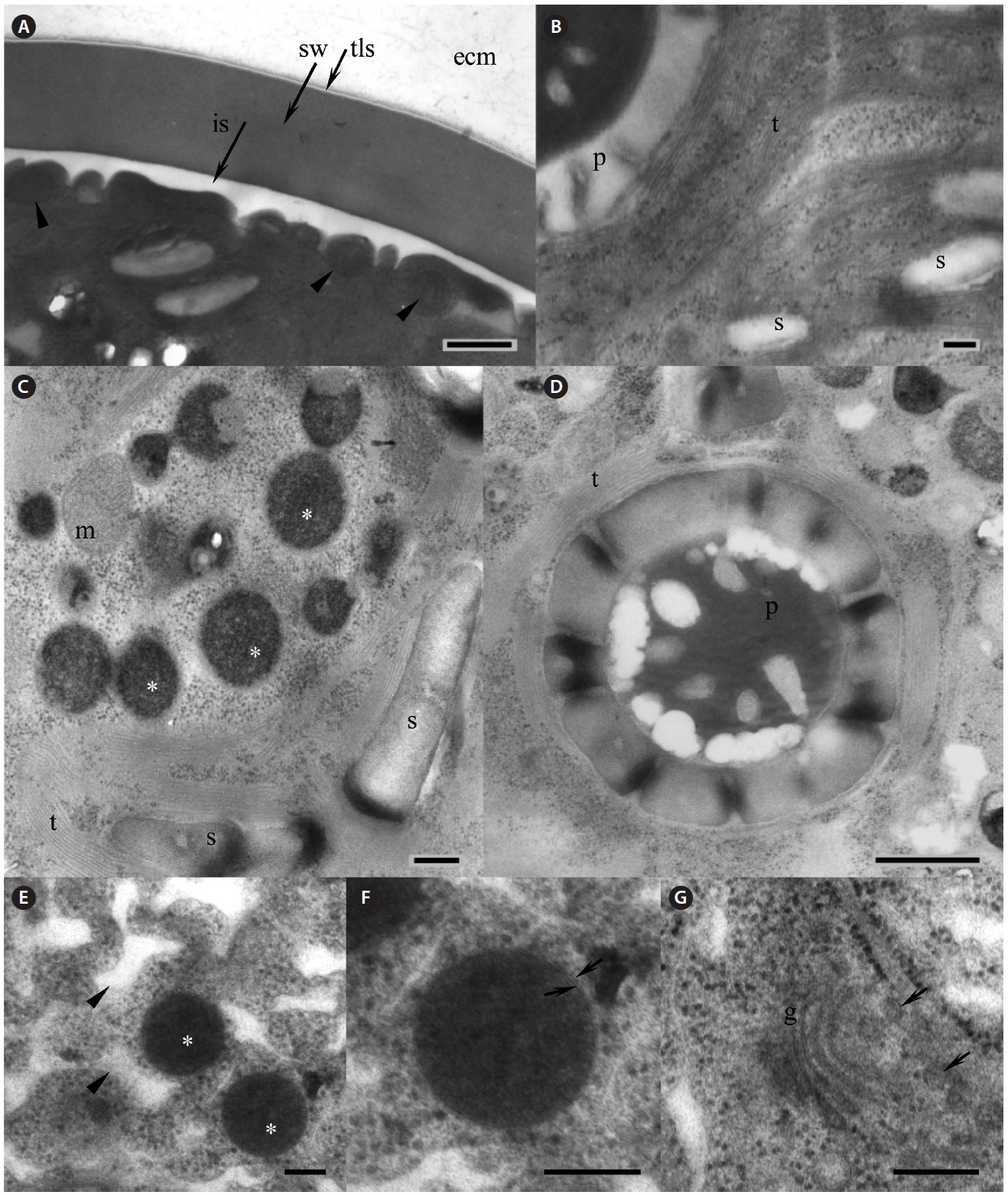
Our strain is predominantly always in non-motile palmelloid stage. Both green- and red-colored spherical non-motile palmelloid cells (Fig. 2A, B & G) are 19.2- 44.8 μm in diameter (28.2 ± 1.9 μm on average) and have a thick cell wall (secondary wall) reaching up to 930 nm thick (Fig. 3A). Some cells are ellipsoidal. The centrally
positioned nucleus was up to 9.5 μm in size, with a large nucleolus (data not shown), and a small Golgi body positioned next to the nucleus was observed (Fig. 3G). Large chloroplast with numerous starch grains occupies the whole cell’s periphery (Figs 2C, D, 3B & C). Chloroplast of young cells contains 2-4 pyrenoids and large old cells have up to 8-12 pyrenoids. No lamellae enter the pyrenoid and the starch sheath is composed of numerous grains (Fig. 3B & D). The thylakoids are stacked in a rather random manner (Fig. 3B-D). Numerous small mitochondria were mostly located beneath the cell membrane (Fig. 3A) and some mitochondria were seen in the cytoplasm (Fig. 3C).
Lipid vesicles packed almost the whole cytoplasmic volume in the cells. Massive lipid accumulation occurred regardless of the pigment accumulation, since green-colored palmelloids (Fig. 2A & B) also contained numerous lipid vesicles (Fig. 3C). In the green palmelloids lipid vesicles were less electron-dense and 200-400 nm in diameter, whereas red palmelloids (i.e., astaxanthin-containing) had more electron-dense lipid vesicles (Fig. 3E & F) of 200-1,000 nm in diameter. The cytoplasm was heterogeneous and segregated (Fig. 3E); however this might have been an artifact of the fixation.
Motile bi-flagellated cells (Fig. 2E) were rarely seen in field-collected samples (4 bi-flagellated cells among several hundred non-motile astaxanthin-containing red cells). Also, they are rarely seen in the laboratory culture. Motile spores have a cup-shaped chloroplast with 1-2 pyrenoids and one large vacuole positioned in the cell center or several smaller scattered vacuoles. Cytoplasmic threads extending from the protoplast to the wall are extremely fine. The cells retain motility for 1 day after release, and thereafter they settle on the substrate and begin to develop palmelloid morphology.
When this strain was first brought from the field, it could live only at 4-6℃; however >80% of the cells were always red-colored. The cells show slow growth at 4-6℃.
[Fig. 3.] Electron microscopy of Haematococcus pluvialis palmelloid cells. (A) Thick cell wall of the palmelloid cell, showing trilaminar sheath (tls, arrow) and smooth secondary wall (sw). Arrowheads point to mitochondria located beneath the cell membrane. (B) Arrangement of the thylakoids in the chloroplast. (C) Numerous lipid vesicles (asterisks) scattered in the cytoplasm of green palmelloid cell. (D) Pyrenoid within a starch-containing chloroplast. (E) Electron-dense astaxanthin-containing lipid vesicles (asterisks) scattered in the heterogeneous segregated cytoplasm (arrowheads) of the red palmelloids. (F) Enlarged lipid vesicle. Arrows point to a membrane surrounding the vesicle. (G) Vertical section through the Golgi body (g). Arrows point to the adjacent Golgi vesicles. ecm, extracellular matrix; is, interspace; m, mitochondrion; p, pyrenoid; s, starch; t, thylakoids. Scale bars represent: A, C & E-G, 200 nm; B & D, 500 nm.
After a period of 860 days in culture at this temperature, we counted only 230,000-866,000 cells in a total volume of 20 mL of BG11 medium, which were progeny from 1 cell.
Over the subsequent period of laboratory culture for 1.5 years, this isolate was gradually acclimated to grow at 15℃. When cultured at 15℃ under 30-35 μmol photons m-2 s-1, palmelloids are mostly green-colored. This strain reproduces efficiently by means of motile flagellated spores after 1-2 days-long exposure to 20℃; however it is not able to live at this temperature for more than 2-3 days. Astaxanthin is accumulated by stressing cells grown at 15℃ under 30-35 mol photons m-2 s-1 with low temperature between 4-10℃ under 30-35 μmol photons m-2 s-1. Under cold stress, efficient astaxanthin production occurs even in the low light (approximately 1.7-9 μmol photons m-2 s-1). Red globular regions appear towards the center of palmelloid cells (Fig. 2F), and after several days of the same treatment cells appear completely red (Fig. 2G).
The cold-tolerant strain of Haematococcus pluvialis discussed in the present study was collected only from one site on Blomstrandhalvøya Island, which was a small rock pool located at a distance of several meters from the sea, but we did not find it in other surveyed areas on this island and in the vicinity of Ny-?lesund (Kim et al. 2008, 2011). Droop (1961) also noted that H. pluvialis typically inhabited rock pools, often, although not necessarily, within a few feet of the sea. We previously suggested anthropogenic introduction of some microalgae to this Arctic area (Kim et al. 2011). It is noteworthy that no Haematococcus microalgae were recorded from this region before and in general H. pluvialis inhabits temperate climate. When defining area of species distribution one should consider that it is limited by the temperature for maximum and / or optimum reproduction and not by the temperatures for survival. Our strain of H. pluvialis from Blomstrandhalvøya reproduces efficiently at 15-20℃, while the average summer temperature of Svalbard reaches 4-6℃. If introduction supposedly took place, it is not a recent event and should have occurred at least a number of years ago, since the cells had to go through several generations of their life cycle to adapt to the harsh Arctic environment. The unfavorable environment might have influenced the specifically low growth rate of this strain. Further work is required to overcome the drawbacks in its vegetative culture to increase the growth rate and culture productivity.
Knowledge of the morphology and life cycle of H. pluvialis becomes increasingly important due to the interest in this organism as a biotechnological source of astaxanthin. However, studies on its morphogenesis and ultrastructure proceed slowly in comparison to other microalgae. The palmelloid cells have so thick cell wall that chemical fixation and embedding prior to ultrathin sectioning and electron microscopy becomes almost impossible (Hagen et al. 2002). The cells of our strain were notoriously difficult to fix for TEM using chemical fixation method and cryofixation. These treatments resulted in turgor breakage and complete disintegration of the cellular contents, and formation of massive ice crystals rupturing the organelles, respectively. Only high-pressure freezing with the following cryofixation was partially successful to preserve some cell structures more or less intact. Our TEM results were conclusive with Hagen et al. (2002), showing presence of thick and smooth secondary wall, which is believed to be mainly composed of granulose non-fibrillar mannan I, a trilaminar sheath, and a layer of extracellular matrix in the palmelloid cells. The cytoplasm of the green palmelloids was packed with lipid vesicles, implying that abundant lipid accumulation occurs prior to the accumulation of the astaxanthin inside the vesicles.
Phylogenetic relationships within Haematococcus have been poorly resolved and only four members of the genus have been sequenced to date, including H. droebakensis Wollenweber, H. lacustris (Girod-Chantrans) Roststafinski, H. pluvialis, and H. zimbabwiensis Pockock, resulting in 1, 11, 211, and 6 nucleotide sequences of different products for each species deposited in the GenBank, respectively (National Center for Biotechnology Information 2013). Also, nucleotide sequences of different products for 3 unidentified Haematococcus sp. have been deposited in the GenBank (National Center for Biotechnology Information 2013). Although H. pluvialis is the most often used for sequencing, no comprehensive study was undertaken to resolve relationships among different strains registered in the database. The name H. pluvialis might have been commonly attached to any red-colored palmelloid Haematococcus, while different similar looking isolates might potentially be numerous new cryptic species. For instance, species H. lacustris is also synonymized with H. pluvialis (e.g., Hagen et al. 2002, Pentecost 2002), however they have a principal morphological difference such as H. lacustris is predominantly always in motile flagellated stage (Pocock 1960), whereas H. pluvialis is predominantly always in non-motile palmelloid stage. Until more comprehensive phylogenetic analysis on different strains of H. pluvialis from around the world is complete, we suggest attributing our strain to this species based on current phylogenetic result. Morphologically, it is not very different from H. pluvialis to support its separation as a distinct species; however it significantly differs in physiology. Unlike other described strains of H. pluvialis, our strain is adapted to live and produce astaxanthin in the low temperature (4-10℃). Most commonly cultured species of algae are able to grow at temperatures between 16 and 27℃ and the optimal growth temperatures are usually in the range of 18-20℃ (Chen et al. 2009). As evident from the numerous references on H. pluvialis, its usual growth temperature for commercial cultivation is between 20-27℃. Our psychrophilic strain is therefore an exception and can potentially be used for astaxanthin exploitation in colder climates.