A morphologically distinct lineage within the Bostrychia moritziana-B. radicans species complex is described as a new species. Bostrychia anomala has thalli with branched monosiphonous filaments with apical cell divisions. The species has terminal tetrasporangial stichidia, each subtending cell bearing tetrasporangia with 2 cover cells. Discharged spores divide transversely, the lower cell first forming a narrow rhizoid and the upper cell forming a monosiphonous shoot. Females have subterminal procarps and males have terminal spermatangial stichidia. Carposporophytes are spherical. Isolates in culture show a pattern of cell death not associated with injury, reminiscent of programmed cell death. Bostrychia anomola shows cell death at intervals along the filaments resulting in division of adjacent cells on either side of the dead cell re-joining the filament; cell division of only one adjacent cell resulting in branching at that site; or filaments fragmenting at the cell death point with adjacent cells forming new apical cells, a means of thallus propagation. The cell death pattern could be a method of filament propagation in the mangrove environment where sexual reproduction is rare.
The biology of red algae in the genus
The Rhodomelaceae, to which
Programmed cell death (PCD), also known as apoptosis, while a well studied phenomenon in many organisms including plants (Van Doorn 2011) is poorly studied in red algae but it has been alluded to in the death of hair cells and trichoblasts, as well as formation of regular thallus perforations (Garbary et al. 2012). Cell repair, after artificial cell wounding, has also been studied in red algae (Kim et al. 1988, 1995, Kim and Fritz 1993). Kim et al. (1988) described three types of cell repair patterns (elongation type, fusion type, and non-fusion-type). A combination of both PCD and repair response has not previously been documented in red algae.
During our investigations of algae in the
Methods for collection, isolation and maintenance of cultures are presented in West and Zuccarello (1999) and West (2005). To promote slower growth low nutrient levels were used initially (2 mL modified-Provasoli-media [MPM] enrichment per liter of sterile seawater) and 18-22℃, low light levels, <5 μmol photons m-2 s-1 cool white fluorescent or LED lighting at 12 : 12 LD daily cycle. The seawater was adjusted to 30 practical salinity units (psu) with Milli-Q water (Millipore Corp., Billerica, MA, USA). Careful observations with a dissecting stereomicroscope were made and short (1-4 mm) apices checked for contaminating epiphytes were excised with micro-forceps and subcultured in 50 × 70 mm crystallizing dishes containing 30 psu seawater medium with 10 mL MPM enrichment per liter. Most of these unialgal cultures were maintained in 18-22℃, 5-20 μmol photons m-2 s-1 cool white fluorescent or LED lighting at 12 : 12 LD daily cycle.
Photography was done using bright field optics on a Zeiss GFL microscope (Carl Zeiss, Jena, Germany) with a Canon G3 camera (Canon, Tokyo, Japan) and Photoshop CS to capture images. Aniline Blue stain (0.02%) in 50% Karo syrup was used to stain tetrasporangial stichidia of thalli fixed in 5% formaldehyde-seawater. All other images were of living specimens.
The methods for DNA extraction and amplification of
the freely available Bioportal (http://www.bioportal.uio.no).
The
>
Bostrychia anomala J. A. West, S. Loiseaux de Goer & G. C. Zuccarello sp. nov.
Uniseriate filaments entirely monosiphonous with apical cell divisions, branching not apically derived but formed by lateral bud cells from intercalary cells or by single intercalary cell deaths followed by lateral growth of an adjacent cell. Tetrasporangial stichidia terminal with 2-4 segments, each segment bearing 2-3 tetrasporangia with 2 cover cells. Females with subterminal procarp and 3 sets of pericental cells around the axial cell. Terminal cystocarps with a distinct ostiole. Males with terminal spermatangial stichidia. Cell death occurs at variable intervals along the filaments and results in: 1) ‘non-fusion division’ by adjacent cells on both sides rejoining the filament; 2) cell division of only one adjacent cell resulting in branching at that site; 3) filament fragmentation forming new thalli.
Type localities. Polysiphonous
Holotype. Dried specimen from culture 4613. NSW 904958.
Paratype. Dried specimen from culture 4588. NSW 904960.
Holotype and paratype cultures. Isolates 4588 and 4613 are available at the Korean Marine Plants Collection, Chungnam National University, 220 Gung-dong, Youseong-gu, Daejeon 305-764, Korea.
>
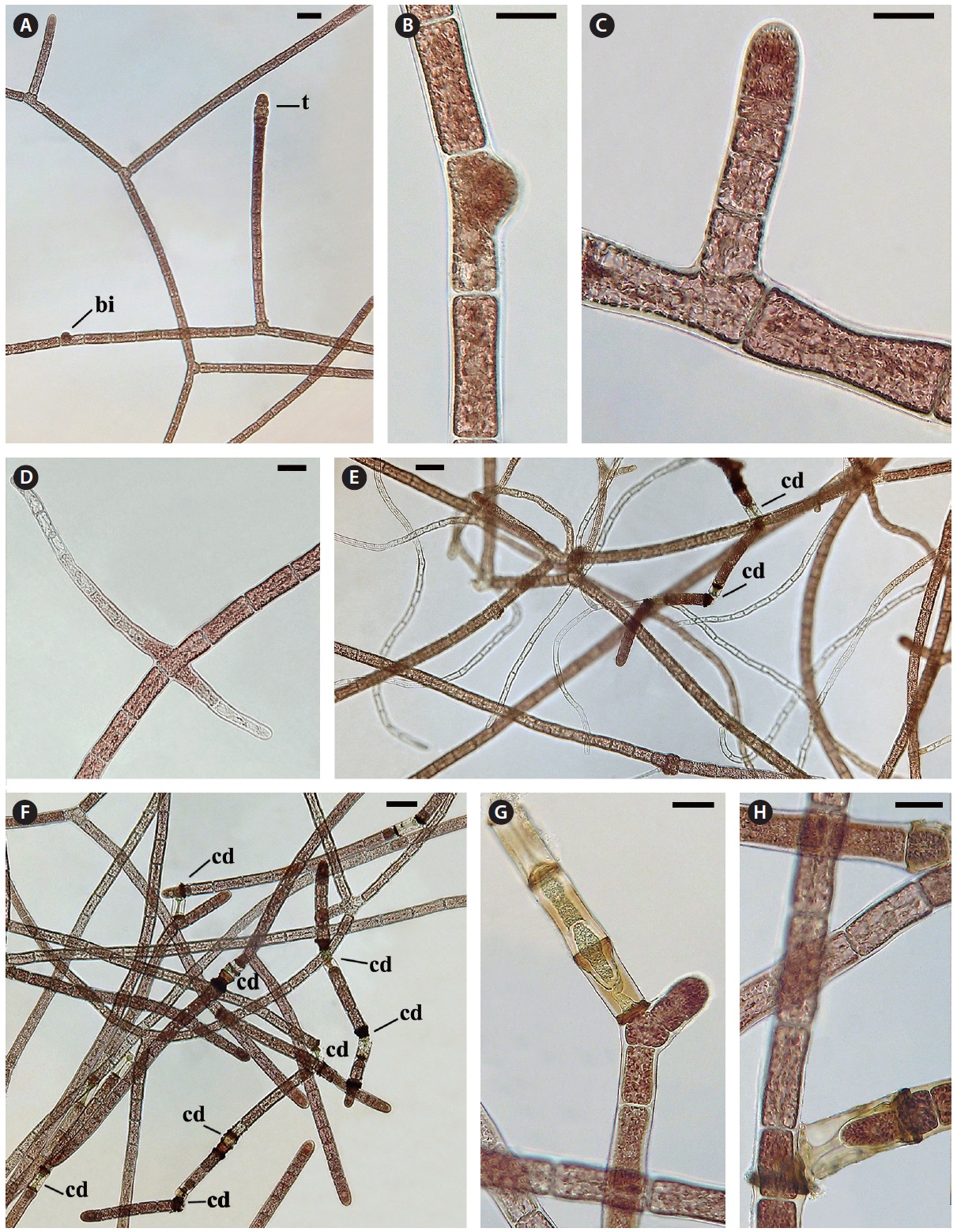
Filament growth and branching
The monosiphonous filaments are quite uniform with a cell diameter of 28-30 μm and cell length varying from 48-80 μm. Filament growth is by apical cell division (Fig. 2C). Each cell has a central nucleus, 8-10 μm in diameter, suspended in an axial cytoplasmic strand. Chloroplasts are purple to pink, discoid to oblong or polygonal (3-5 μm) and positioned in the peripheral cytoplasm and in the central axial strand. Lateral branching does not occur at the apex as seen in polysiphonous
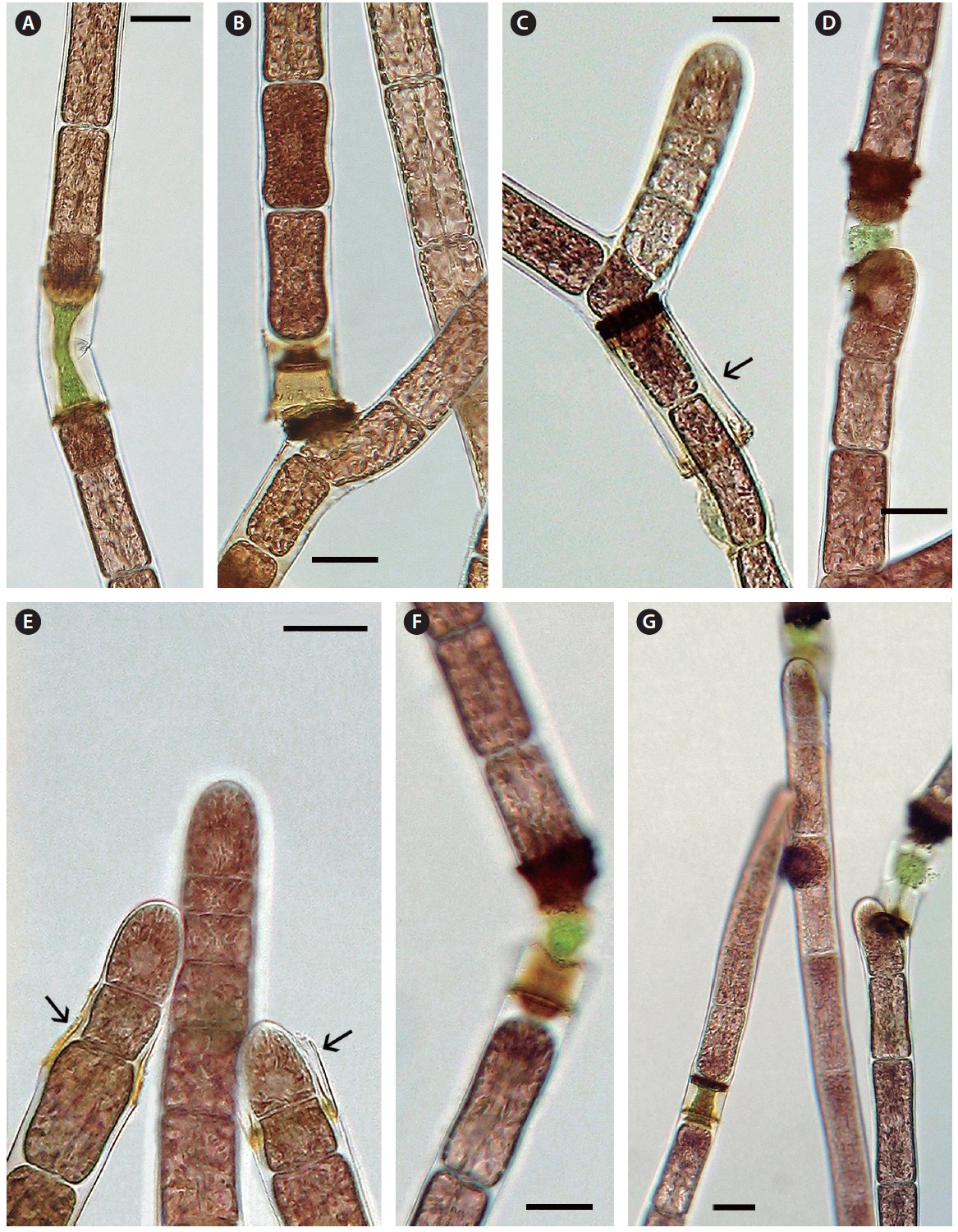
Cell death and repair. Cell death is evident throughout the monosiphonous filaments. This phenomenon has been noticed over several years in these isolates and is a regular phenomenon. The regularity and numbers of cell death indicates that this is a due to a developmental process and is not due to injury. When a cell protoplast collapses a homogeneous green to brown colour appears within the dead cell (Figs 2G, H & 3A-G). A dark brown collar is seen at either end of the dead cell (Figs 2G & 3A-G). Within 1-2 days one or both adjacent cells elongate slightly (Fig. 3A), change from a vegetative cell to a meristematic cell with a darker red and denser protoplast that divides transversely and grows into the dead cell space (Fig. 3A-C). These new cells are similar to apical cells in the monosiphonous filaments. Cell death may also take place in a cell at filament branch nodes (Fig. 3B). The cell adjacent to the dead basal cell of the lateral branch elongates forming a new cell that grows through the dead cell (Figs 2H & 3B). The other adjacent cell in the main filament often does not change (Figs 2H & 3B).
In other cases, possibly when the position and distance between the adjacent cells change, the apical cell on one side will curve away from the axis of the filament (Fig. 3D) and form a new branch that can break away forming a new filament. When a filament breaks at a dead cell both cells adjacent to the dead cell become shoot tips of new filaments (Fig. 3E). Also if two or more cells in a sequence die the adjacent live cell divides, elongates laterally and forms a new shoot apex (Fig. 2G).
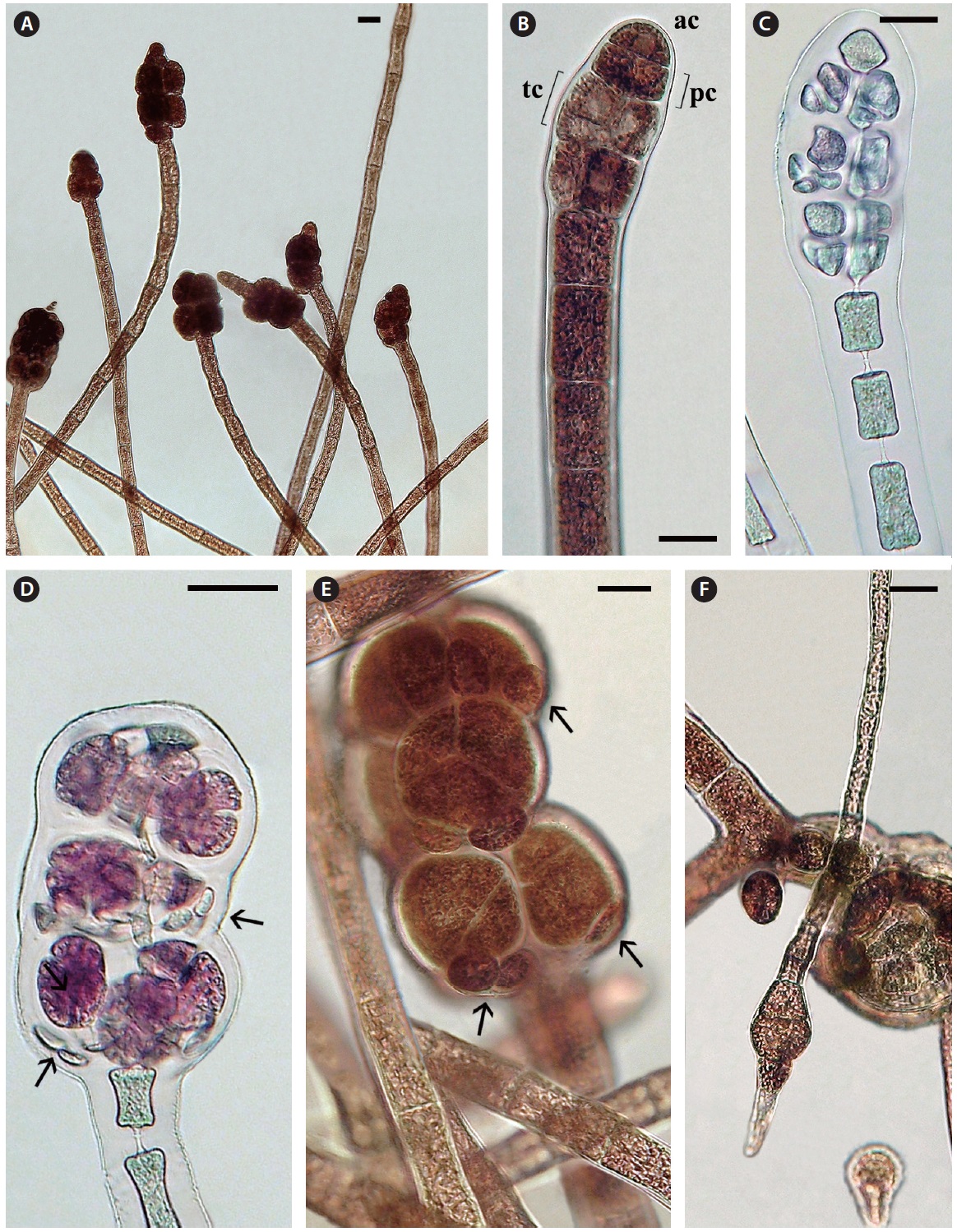
Tetrasporophytes. Terminal tetrasporangial stichidia are numerous (Fig. 4A). Development is initiated with the formation of a series of pericentral cells by 2-4 axial cells, adjacent to the apical cell (Fig. 4B & C). Often the apical cell resumes cell divisions and vegetative growth so that mature stichidia become intercalary (Fig. 4A). The pericentral cells divide transversely forming tier cells (Fig. 4B) like those of normal polysiphonous
to form 1-2 cover cells that project out from the lower part of each mature tetrahedrally divided sporangium (68-77 μm diameter) (Fig. 4D & E). Discharged spores are about 30-36 μm diameter and the spores divide transversely once, the lower cell first forming a slightly narrow rhizoid and the upper cell secondarily forming a monosiphonous shoot (Fig. 4F). No pericentral cells were observed during vegetative growth of any sporelings.
Female gametophytes. Carpogonial branches develop on a subterminal cell (Fig. 5A) of the monosiphonous branches and the shoot elongates if no fertilization occurs and the procarps become intercalary (Fig. 5B & C). The female thalli did not persist in culture so consequently it was not possible to investigate the further details of procarp-carposporophyte development but it appears that the carpogonial branch base is partially enclosed by two sets of prominent sterile cells (Fig. 5C). Mature cystocarps of about 350 μm diameter were observed only twice with a well developed pericarp and terminal ostiole, producing about 40 carpospores (Fig. 5E & F).
Male gametophytes. Terminal spermatangial stichidia are about 50 μm wide and up to 400 μm long (Fig. 5D). These stichidia are quite similar to the spermatangial stichidia borne on monosiphonous laterals of some polysiphonous
The monosiphonous thalli of isolate 4588 showed no spore reproduction in field or culture specimens. Propagation in culture resulted entirely from cell death and breakage along the filaments producing many short filaments. This isolate has slightly smaller cells than isolate 4613 (22-26 μm diameter and 46-53 μm long).
The consistent morphology of these unusual isolates and their phylogenetic position as a well-supported lineage within the
Isolates 4588 and 4613 were the only field samples we observed to be monosiphonous and have remained so in culture for 7 years. The molecular evidence and reproductive characters were essential in linking
Isolates of Bostrychia moritziana-B. radicans in which monosiphonous filaments are occasionally seen
A most unusual feature of
When filaments do repair they mostly follow a pattern, recognized as the “non-fusion-type” as proposed by Kim et al. (1988). Cell division is initiated in the cells adjacent to the dead cell and the elongating cells abut within the cytoplasmic space of the dead cell. The cells newly in contact must be able to adhere as the filament is not weaker at this location. Both PCD and wound repair are phenomena in red algae that warrant increased scientific research.
Our data clearly indicates that an evolutionary lineage within the
![Maximum-likelihood topology of rbcL sequences of members of the genus Bostrychia. Outgroups removed for clarity. The bootstrap values for maximum-parsimony trees (MP) (> 50%; right) and maximum-likelihood trees (ML) (≥ 50%; left) are given on each branch, and strongly supported branches (MP-bootstrap [BS] and ML-BS ≥ 95%) are indicated by asterisks. Bayesian inference values over 0.95 are shown by thicker branches. For further information on samples see West et al. (2006), Zuccarello and West (2006), and Zuccarello et al. (2012), except for newly derived sequences (underlined).](http://oak.go.kr/repository/journal/12109/JORHBK_2013_v28n2_161_f001.jpg)