In recognition of the current energy crisis, alternative energy sources are currently being explored and developed at an increasing pace; biofuels in particular have received a considerable amount of attention over the past few years. Conventional biofuels, such as bioethanol and biodiesel, are produced primarily from cereal crops and oil seeds. However, the mass production of crop-based biofuels has resulted in serious side effects, such as increases in food prices, deforestation, and carbon emissions (Sims et al. 2010). Thus, algae-based biofuels have been recognized as an attractive option, as they use minimal land resources (Chisti 2007) and do not compete with food production (Huang et al. 2010). Additionally, algaebased biofuels have several other advantages, including rapid growth rates (Schenk et al. 2008), higher lipid contents (Hu et al. 2008), and higher CO2 uptake rates (Jorquera et al. 2010) relative to other energy crops. Considering these advantages, microalgae biofuels have been recognized as the only current renewable source of liquid transportation fuel which is compatible with the existing engines and distribution systems (Schenk et al. 2008). Microalgae, including cyanobacteria, have been reported to generate a variety of lipids, hydrocarbons, fatty alcohols, and other complex oils (Metzger and Largeau 2005, Guschina and Harwood 2006, Schirmer et al. 2010, Tan et al. 2011). Although not all algal oils are suitable for biodiesel production, a number of microalgae have been suggested to be satisfactory for this purpose (Chisti 2007, Xin et al. 2010).
However, there are still a number of technical barriers which must be overcome prior to their immediate application to the production of such fuels, and one of the major challenges remaining in algae biodiesel production is the reduction of the cost of lipid extraction, which accounts for up to 90% of the total energy consumption involved in the production of algae biodiesel (Lardon et al. 2009). Algal biodiesel is usually obtained by transesterifying algal oil triglycerides with an alcohol in the presence of an acidic or basic catalyst (Meher et al. 2006, Demirbas 2007, Johnson and Wen 2009). Methanol is the most commonly used alcohol in this process due to its low cost (Demirbas 2005), yet it has been reported that methanol was responsible for 47% of the total energy consumption in biodiesel production (De Souza et al. 2010). Recent work by Schirmer et al. (2010) showed that some cyanobacteria could naturally produce alkanes such as pentadecane (C15H32) and heptadecane (C17H36). As petroleum-based diesel is made up of a mixture of alkanes with 8 to 20 carbon atoms, these cyanobacteriaderived alkanes can be directly used as biodiesel without requiring any chemical conversion, and thus, may serve as a possible candidate for the replacement of fossil fuels.
In this study, we isolated a filamentous cyanobacterium,
>
Sample collection and isolation
Samples were taken from puddles of icy water near rice paddies (35°50' N, 128°50' E) in Jillyang-eup, Geyongsan City, South Korea in January 2012. A few micrograms of cyanobacterial mats were used to inoculate 100 mL BG- 11(+) medium containing nitrate (Rippka et al. 1979) with 250 μg mL-1 cycloheximide (Sigma, St. Louis, MO, USA).
[Table 1.] Result from BLAST search using the 16S rRNA sequence of Phormidium autumnale KNUA026

Result from BLAST search using the 16S rRNA sequence of Phormidium autumnale KNUA026
The flasks were incubated on an orbital shaker (Vision Scientific, Bucheon, Korea) at 160 rpm and 15℃ under cool fluorescent lighting (approximately 70 μmol photons m-2 s-1), until growth was apparent. An aliquot of the brown-colored biomass was taken by pipetting, and was sonicated for approximately 3-5 s using an ultrasonic cell disruptor (Model 550; Fisher Scientific, Pittsburgh, PA, USA). The filaments were then transferred onto BG-11 agar plates containing 100 μg mL-1 of meropenem (Yuhan Pharmaceuticals, Ochang, Korea) and were incubated in the dark for 24 h to eliminate contaminating bacteria (Choi et al. 2008). The culture was then inoculated onto fresh BG-11 agar plates and incubated for 14 days under a light : dark cycle (16 : 8 h) at 15℃.
>
Morphological and molecular identification
The isolate was grown in BG-11(+) medium for 21 days. Live cells were harvested by centrifugation at 3,000 ×g for 5 min, washed with sterile distilled water, and inspected at 400× magnification with a Zeiss Axioskop 2 light microscope (Carl Zeiss, Standort Gottingen, Vertrieb, Germany) equipped with differential interference contrast (DIC) optics.
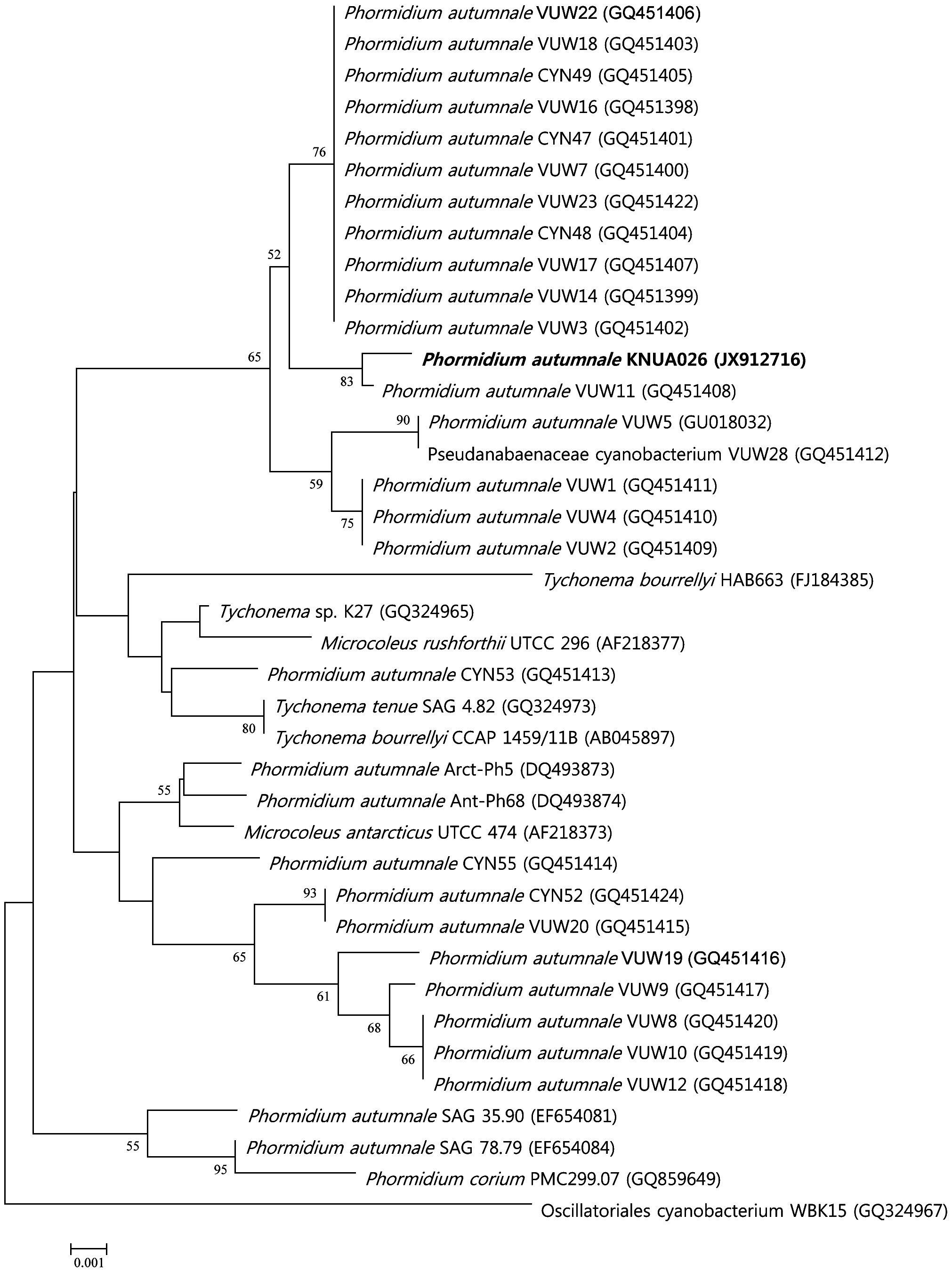
For the amplification of 16S rRNA gene fragments, the primer set, CYA106F and CYA781R(a) and CYA781R(b), as described by Nubel et al. (1997) was used. Synthesis of the primers used in this work and DNA sequencing were performed at the Macrogen facility (Macrogen, Seoul, Korea). The DNA sequence obtained was submitted to the National Center for Biotechnology Information (NCBI) database under the accession no. JX912716 (Table 1). Phylogenetic analysis was performed using the software package MEGA version 4.0 (Tamura et al. 2007), using the neighbor-joining method (Saitou and Nei 1987) with 1,000 bootstrap replicates (Felsenstein 1985).
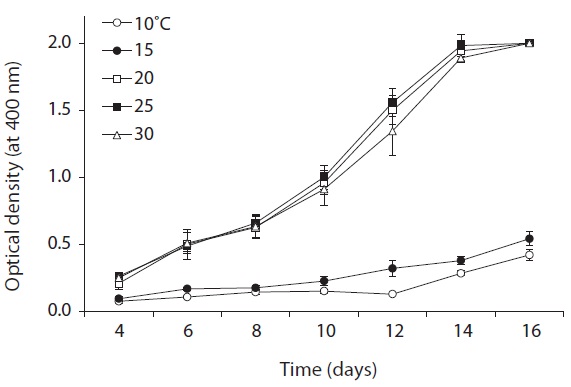
All the physiological tests were performed in 5-mL test tubes containing BG-11(+) medium in triplicate. Survival and growth of KNUA026 cells maintained at 10, 15, 20, 25, and 30℃ (at intervals of 5℃) were examined to identify
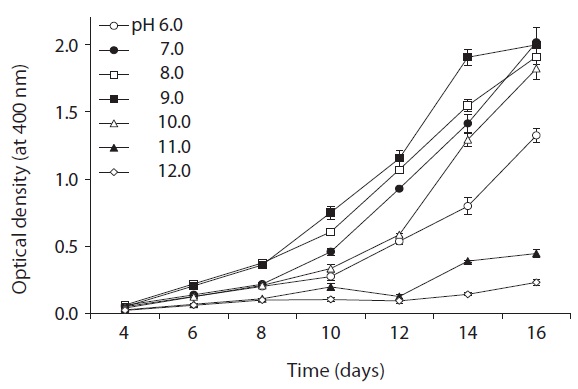
the optimum temperature for culturing. An acidity tolerance test was carried out over a pH range from 6.0 to 12.0 (at intervals of pH 1.0 unit). Each test tube was homogenized by sonication for approximately 3-5 s on an ultrasonic cell disruptor, and the cyanobacterial density was then determined by measuring the turbidity at 400 nm on an Optimizer 2120UV spectrophotometer (Mecasys, Daejeon, Korea). Growth rates were expressed as μ = (ln N2 - ln N1)/(t2 - t1), where N2 and N1 are the value of optical density during the period of exponential growth at times t2 and t1, respectively.
>
Lipid extraction and gas chromatography / mass spectrometry (GC / MS) analysis
To simulate the commercial production of microalgaebased biofuels and to obtain sufficient biomass for analysis, strain KNUA026 was inoculated into 16 L of BG-11(+) medium in an 18-L transparent polycarbonate bottle in triplicate. The cultures were autotrophically grown at 25℃ with a flow of air bubbles at a rate of approximately 2 L min-1 under cool fluorescent lighting (approximately 70 μmol photons m-2 s-1) with a light:dark cycle of 16 : 8 h. After incubating for 21 days, cyanobacterial cells were harvested and the fatty acid composition was analyzed by GC/MS (GCMS-QP2010 ultra; Shimadzu, Tokyo, Japan) according to our previous publication (Yeo et al. 2011).
>
Photobioreactor (PBR) optimization experiments
To explore suitable PBR configurations for the largescale cultivation of
>
Identification of the isolated cyanobacterium
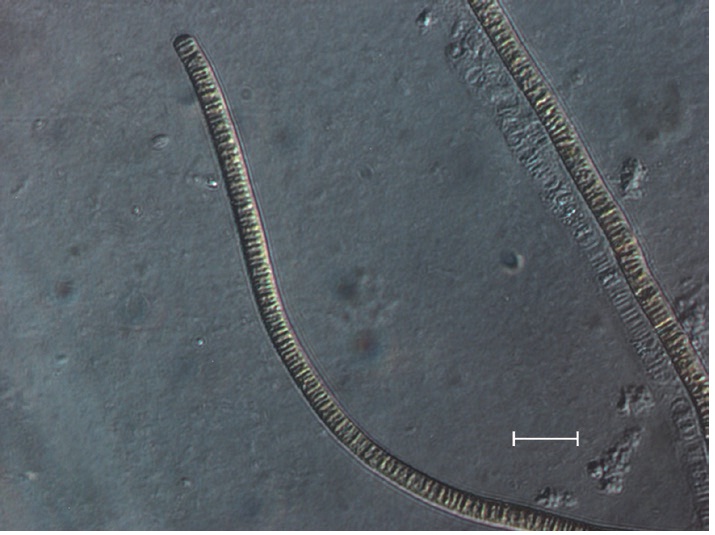
The isolate was an unbranched and filamentous cyanobacterium (Fig. 2). Trichomes were straight, 3.0 to 7.0 μm wide, composed of cylindrical and slightly barrel- shaped cells and densely layered trichomes readily formed macroscopically visible mats up to several cm in diameter (Fig. 3). The cross walls were clearly visible, end cells were widely rounded, and cell contents were brownish.
No heterocytes or akinetes were found. According to the 16S rRNA sequencing data (Table 1), the isolate had a 99% sequence homology with
>
Physiological properties of Phormidium autumnale KNUA026
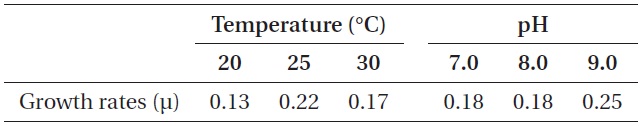
As shown in Fig. 5, strain KNUA026 showed good growth at 25-30℃ and the cells were able to grow and survive at 10 and 15℃, but delayed growth was observed when they were incubated at these temperatures. Even thoughUA026 was isolated from cold freshwater environments, its optimal growth was attained at 25℃. The cyanobacterium also grew well across a wide pH range (pH 6.0-12.0) (Fig. 6) while maximal growth was obtained around pH 9.0. Growth rates (μ) of the isolate at exponential phase are listed in Table 2.
>
Lipid extraction and GC / MS analysis
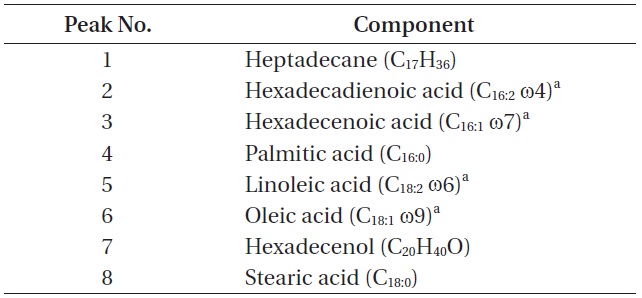
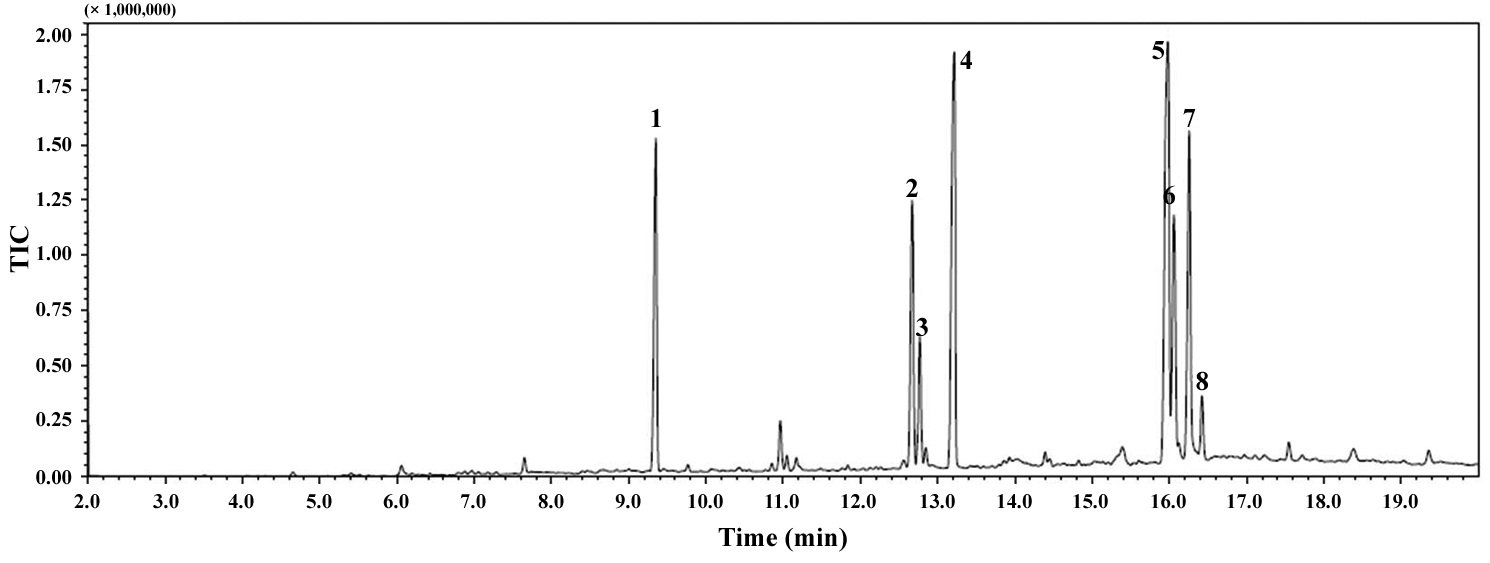
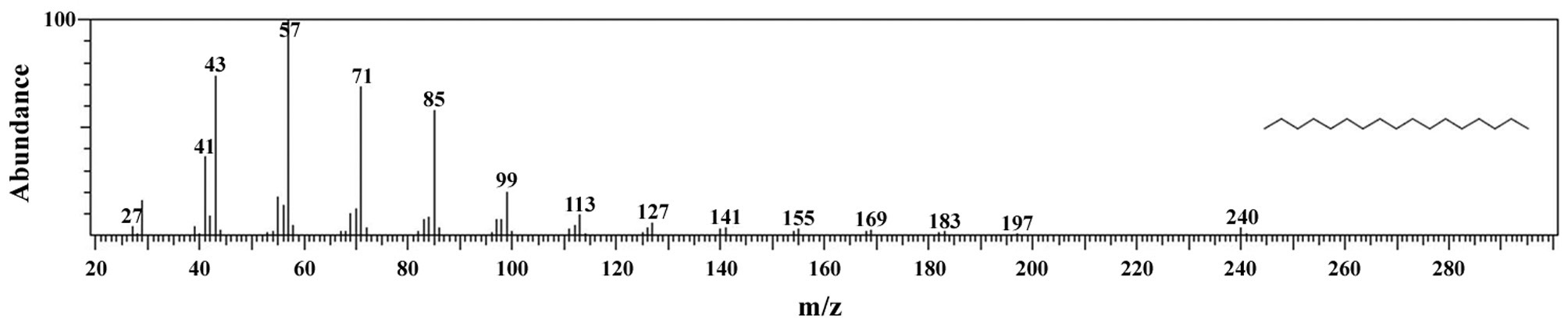
The total lipid content of the isolate was 14.0 ± 1.4% of dry weight and the GC / MS results showed that the KNUA026 strain produced hexadecadienoic acid (C16:2 ω4), hexadecenoic acid (C16:1 ω7), palmitic acid (C16:0), linoleic acid (C18:2 ω6), oleic acid (C18:1 ω9), and stearic acid (C18:0) as its major fatty acids (Table 3, Fig. 7). Interestingly, it was found that a C17 alkane, heptadecane (C17H36, molecular weight = 240) was autotrophically biosynthesized by strain KNUA026 (Fig. 8). The heptadecane content in
dry weight. As heptadecane can be directly used as a biodiesel component without a transesterification step, this isolate may have potential as a cost effective source of biodiesel.
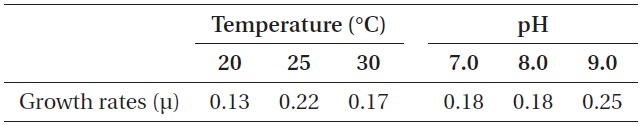
Growth rates (μ) of Phormidium autumnale KNUA026 over a range of incubation temperatures and pH values
[Table 3.] List of cellular components present in Phormidium autumnale KNUA026
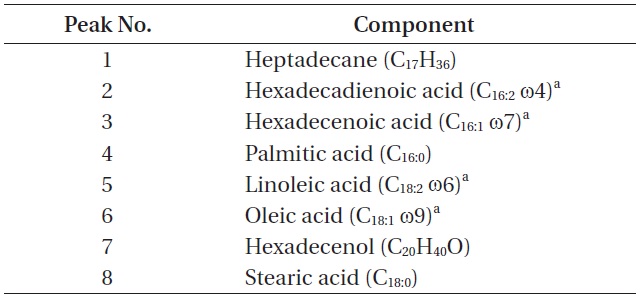
List of cellular components present in Phormidium autumnale KNUA026
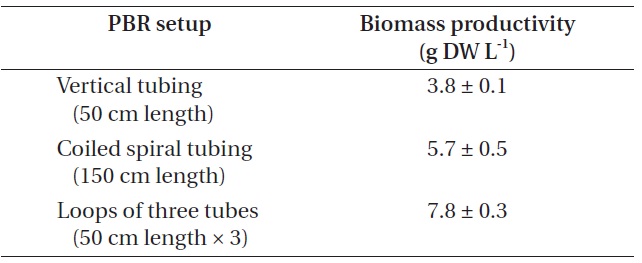
[Table 4.] Biomass production from different photobioreactor (PBR) setup
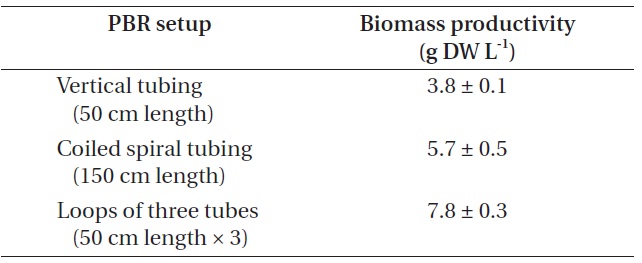
Biomass production from different photobioreactor (PBR) setup
Biomass productivity from each PBR setup was compared (Table 4). In this experiment, the highest biomass was achieved when cultured in the PBR composed of loops of three tubes, as it could provide additional growth surfaces to strain KNUA026. As the isolate grew attached to a surface and formed thick mats, this benthic cyanobacterial culture would be much easier to harvest than a suspended culture. Thus, the isolate may have potential advantages over planktonic species for commercial-scale applications. Future work could address more factors potentially affecting the biomass productivity of
In the present study, a South Korean domestic cyanobacterium,