Galls, or tumors, are unorganized tissue on otherwise normal plants. Galls are usually associated with abnormal cell division patterns and / or cell enlargement (Apt 1988, Scheffer 1997). Galls have not been reported extensively in red algae possibly because they are rare and not considered important, but the deformation of tissue could have fitness consequences for the host. While the cause of all red algal galls is not known several causative agents have been shown or suggested. Cyanobacteria were reported to cause galls in
Galls have also been associated with viruses. Viruslike particles (VLPs) were found in the galls of
We have studied
>
Algal material and laboratory culture
Unialgal culture methods were described in West and Zuccarello (1999) and West (2005). Culture isolates were all maintained at 18-23℃, 12 : 12 LD daily photoperiods, 3-5 μmol photons m-2 s-1 cool white fluorescent or LED lighting, MPM/2 culture medium (30‰ salinity). For faster growth and reproduction cultures were placed in 10-15 μmol photons m-2 s-1 on rotary shaker (70 rpm).
Most isolates used for this research program are now available at the Korean Marine Plants Collection, Chungnam National University, 220 Gung-dong, Yuseong-gu, Daejeon, Korea.
>
Bright field and fluorescence microscopy
Algal nuclei were stained with the DNA-specific fluorochrome DAPI (4′,6-diamidino-2-phenylindole, Sigma- Aldrich, St. Louis, MO, USA) using a heat fixation method. Algal thalli were dipped in 5 μg mL-1 DAPI solution in seawater for 5 min, and then the cover slips were slightly heated over a boiler for a few seconds. After staining, algal thalli were mounted on slides in the DAPI solution and were examined under a UV filter.
Micrographs were taken with Olympus DP50 digital camera affixed to an Olympus BX50 microscope (Olympus, Tokyo, Japan) using Viewfinder Lite and Studio Lite computer programs (Better Light Inc., Placerville, CA, USA) or with a Zeiss GFL bright field microscope (Carl Zeiss AG, Oberkochen, Germany) using a Canon G3 camera (Canon Inc., Tokyo, Japan) and Photoshop CS4 computer program (http://www.adobe.com/au/).
>
Transmission electron microscopy (TEM)
Galls were fixed in phosphate buffered saline (PBS) buffer containing 2% glutaraldehyde at 4℃ for 2 h. The glutaraldehyde was then rinsed out with PBS buffer and the cells were postfixed with 2% osmium tetroxide at 4℃ for 1.5 h. Thereafter, the cells were rinsed out with PBS buffer and were dehydrated in a graded acetone series, embedded in Spurr’s epoxy resin (Spurr 1969) and polymerized overnight in a 70℃ oven (Polysciences Inc., Warrington, PA, USA). Sections stained with uranyl acetate and Reynolds’s lead citrate (Reynolds 1963) were viewed and photographed on a Phillips Bio Twin Transmission Electron Microscope (Phillips Electron Optics, Eindhoven, Netherlands). We were able to carry out TEM studies on only one
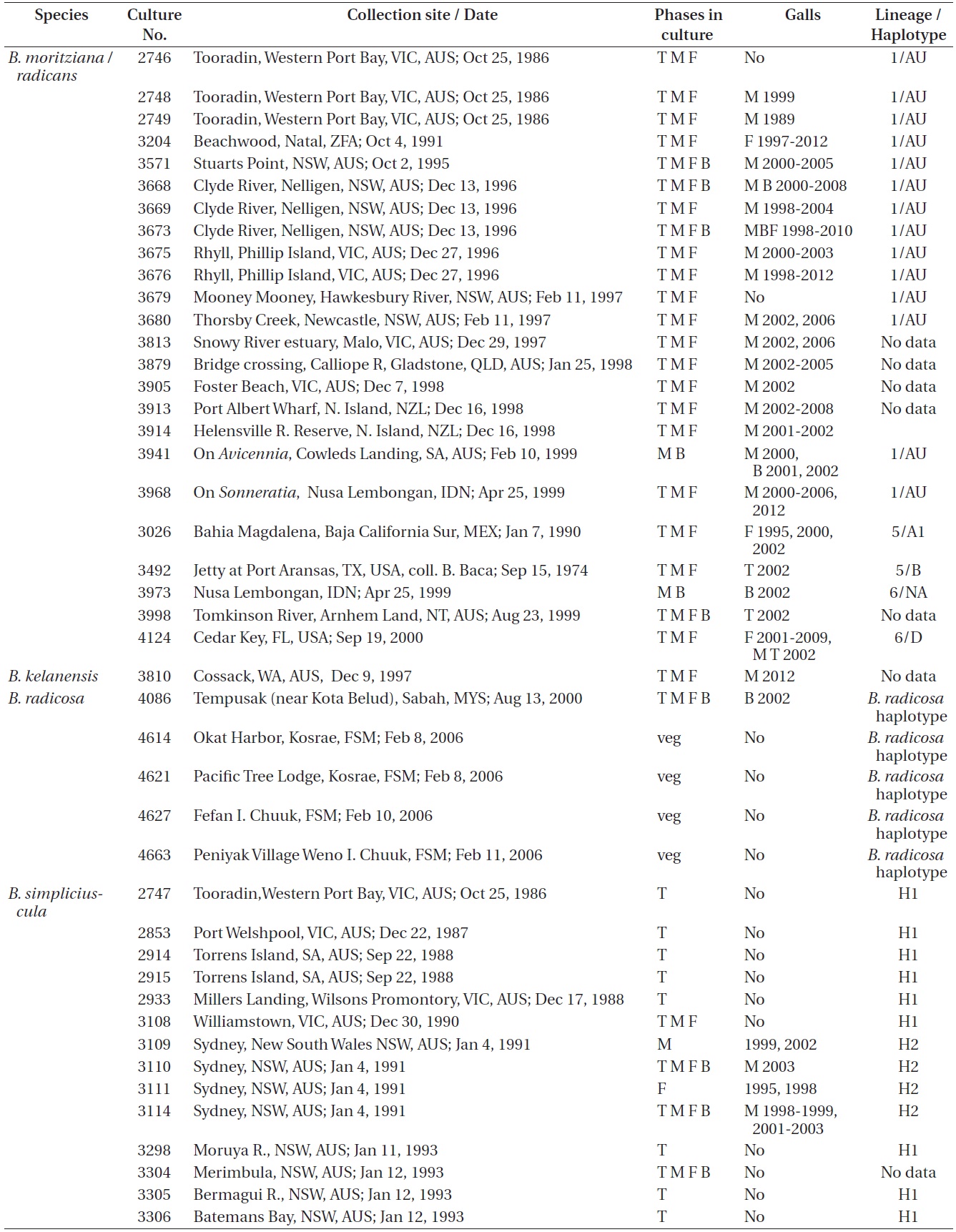
Refer to Table 1 for the time periods (years) in which galls were seen in the various isolates. These galls were predominantly found in isolates from Australia where most isolates were obtained. Only a small percentage of our isolates had galls and they were only observed in
Bostrychia simpliciuscula
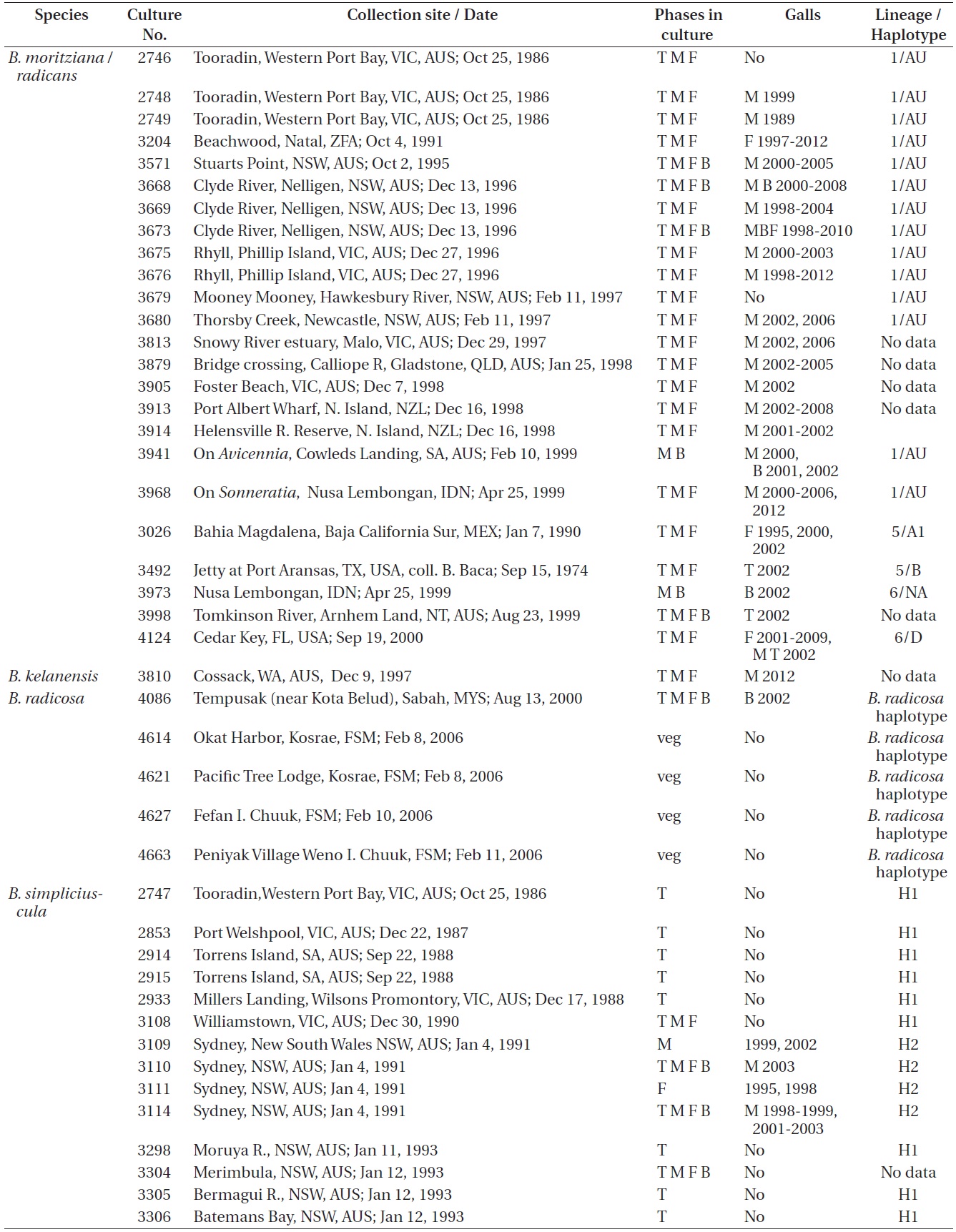
Bostrychia moritziana / radicans, B. kelanensis, B. radicosa, B. simpliciuscula, and B. tenella isolates observed. Rubisco spacer lineages of B. moritziana / radicans and B. simpliciuscula from Zuccarello and West (2003, 2006), Zuccarello et al. (1999) or during this study
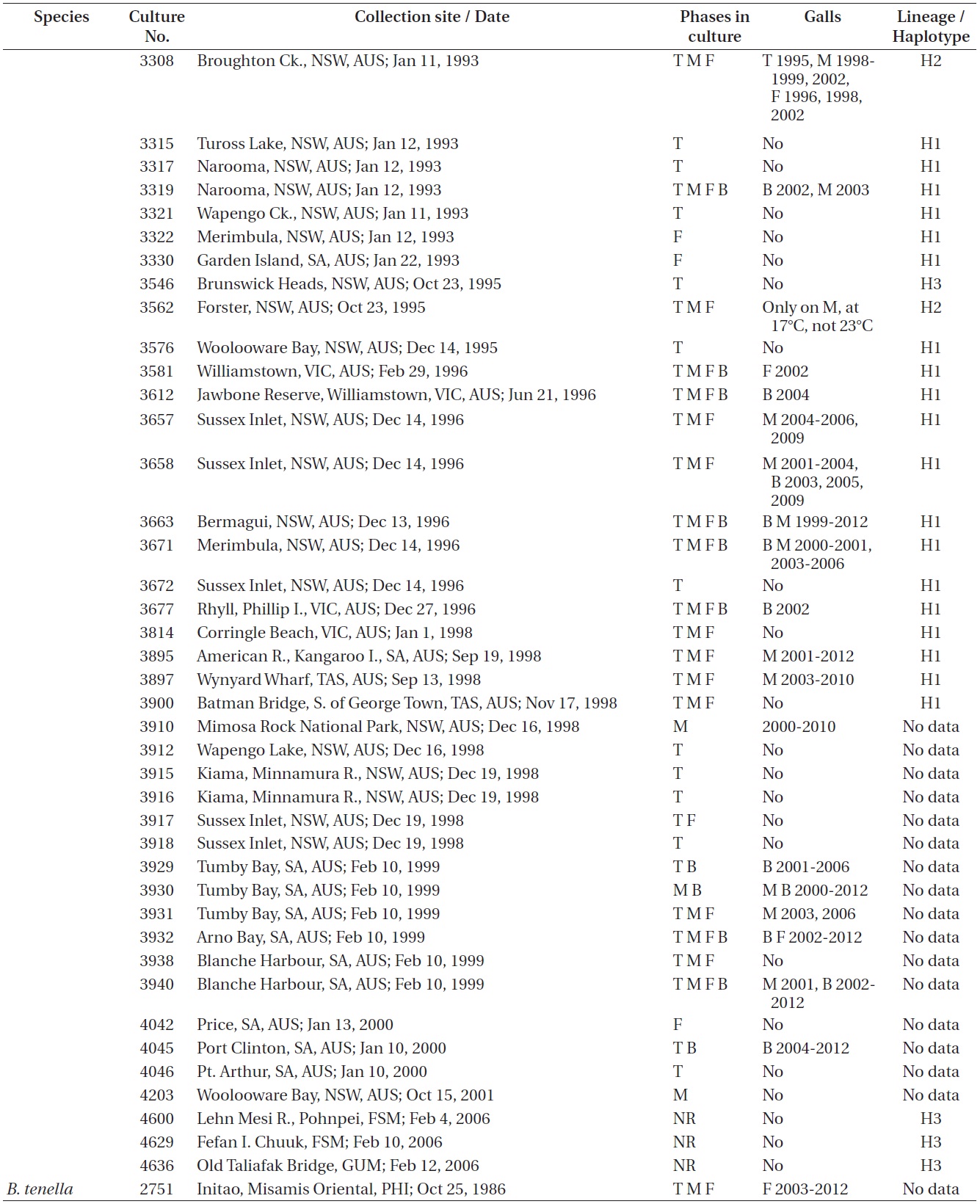
continued
West 2006). Galls were found on 15 of 87 (17%) isolates of lineages H1 and H2, exclusively with Australian isolates. Galls were usually associated with viable spermatangial stichidia, either on unisexual or bisexual plants, although some were evident on the non-reproductive sectors. Tetrasporophytes and female gametophytes occasionally had galls. This has been true over 26 years of culture for some isolates. Galls never developed on young gametophytes, only on reproductively mature gametophytes. On isolate 3562 no galls formed on the tetrasporophytes, females or males grown at 23℃. However, galls developed in the vicinity of spermatangial stichidia of males but not on females or tetrasporophytes when grown at 17℃. Isolate 3562, having numerous galls, was placed in a culture (on a shaker and in brighter light) for 3-6 weeks with the male of another isolate (3108) having a long history without galls. No galls developed on isolate 3108. This is only a partial test of Koch’s postulates (http://en.wikipedia.org/wiki/Koch’s postulates).
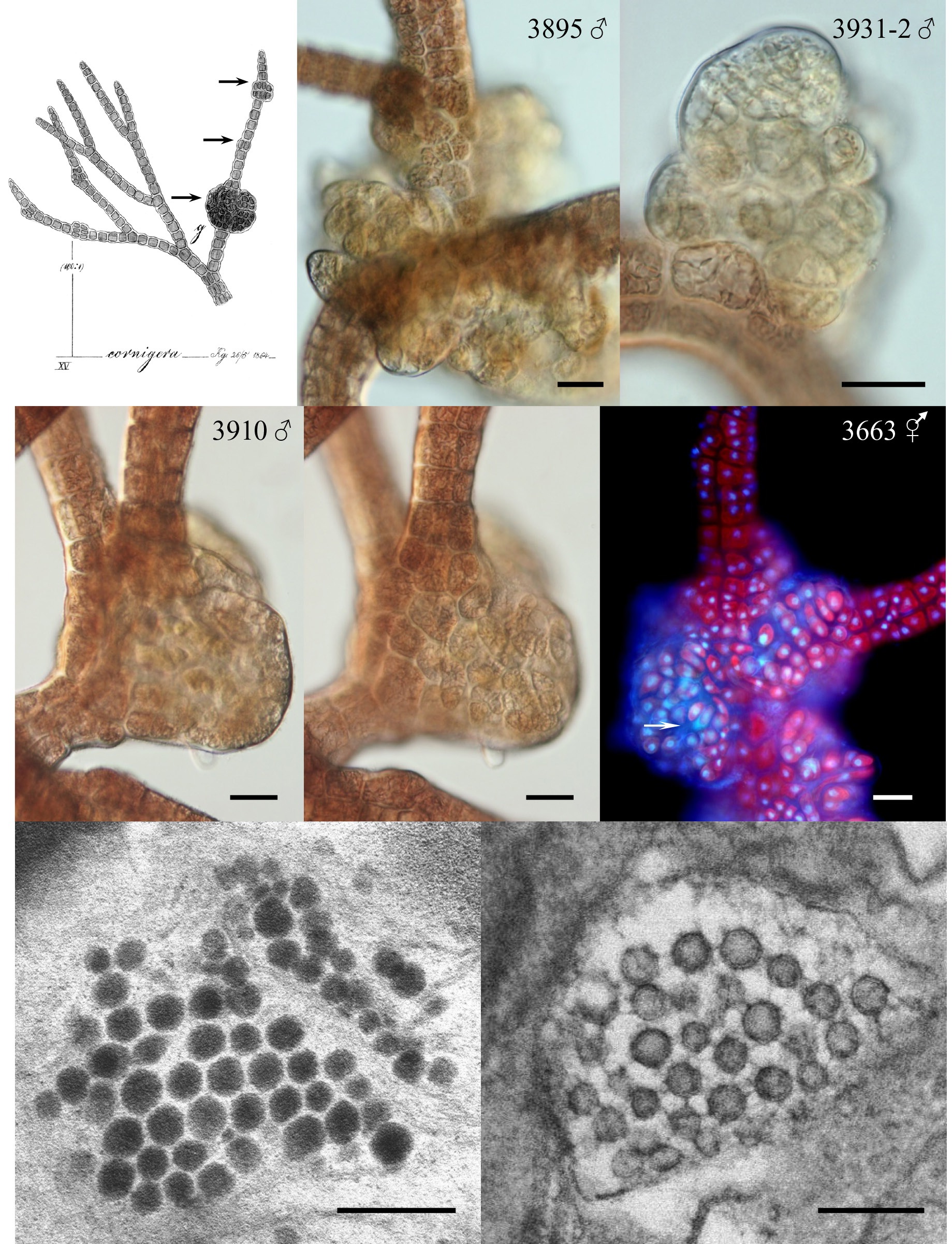
Galls varied in appearance and size. The initial stages appeared as enlarged proliferating cells. Cell divisions appeared random and very different from the polysiphonous tier cell division pattern of the normal host (Fig. 1B-E). The cells in galls were often smaller than tier cells, of various shapes and sizes and had enlarged vacuoles. Gall cells were less pigmented than normal host tissue cells (Fig. 1B). They appeared to have viable nuclei that often were larger than tier-cell nuclei (Fig. 1F). In smaller galls the cells all appeared viable but as the galls expanded and the cell number increased some dead cells were seen (arrow in Fig. 1F). In some larger galls new shoots arose within the cell mass (no photo).
In many isolates galls were present intermittently, however, galls were continuously present on the male and bisexual phases of 3663 (Fig. 1F) from 1999-2012 and on the male phase of 3895 from 2001-2012 (Fig. 1B). Isolate 3932 was unusual because galls appeared on the bisexual or female thalli but not on males from 2002-2012.
Electron microscopic (TEM) observations. While TEM fixation of cellular structure was difficult our micrographs did show VLPs in gall cells of isolate 3895 (Fig. 1G & H). These VLPs are of two distinct morphologies (staining differently) approximately 70-75 nm in size and hexagonal in shape.
>
Bostrychia moritziana / radicans
From 390 isolates of the
In 26 years of culture isolate 2748 produced galls only once (May 1999) on the male spermatangial stichidia and these were very similar in overall structure to those seen in
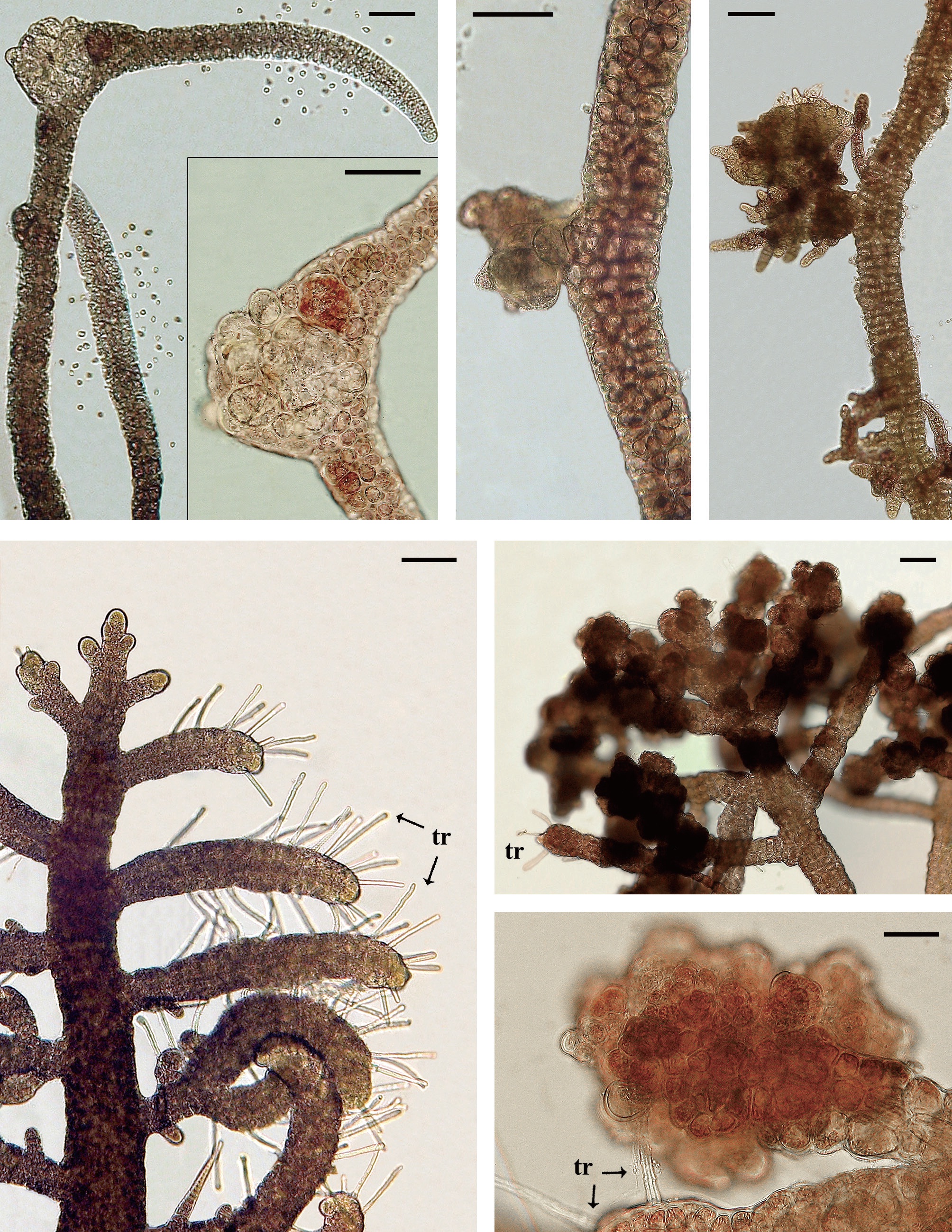
Isolate 3204 female (South Africa) was collected in 1991 and had numerous galls on procarpic lateral branches from 1997-2012 (Fig. 2A & B). Males and tetrasporophytes had no galls. Isolate 4124 female (Florida, USA) was collected in 2000 and developed galls with mostly colorless living cells on the tips of vegetative laterals. Regeneration of viable branches frequently occurred from gall tissue (Fig. 2C & D). Galls were evident for almost 8 years (2001-2009). Males and tetrasporophytes produced galls briefly in 2002.
Galls were observed on 16 males, 4 females, 4 bisexuals, and 3 tetrasporophytes of all isolates in Table 1.
Isolate 4086 (Sabah, Malaysia) was obtained in August 2000 and showed gall formation on the nodes and gametangial sectors of the bisexual gametophytes first in May, 2002. Isolate 4178 (New Caledonia) males developed galls in August, 2012 (Fig. 3A & B). Initially the gall cells enlarged and retained fully pigmented chloroplasts (Fig. 3A) but as the galls matured pale hypertrophied cells with enlarged vacuoles were evident (Fig. 3B). Other isolates of
In 31 isolates of
In 86 isolates of
While the presence of galls is infrequent in
Galls were not observed in laboratory culture on any other
It is noteworthy that almost all species with galls lack cortication except for well-corticated
We have never observed galls on any
The ability of galls to form from healthy tissue separated from other gall tissue, suggests that the causative agent (possibly a virus) may be latent in cells of some
Although not tested extensively three antibiotics (Penicillin G, Ciprofloxacin, and Rifampin) were routinely added to various cultures without any effect on gall presence. During antibiotic treatments various bacteria were also clearly present on the hosts as well.
The greater susceptibility of males and bisexuals to gall formation in
We know very little about causative agents of the galls seen in