The segregation of the genus Neosiphonia Kim et Lee from the large genus Polysiphonia Greville was based on Neosiphonia flavimarina Kim et Lee from Korea (Kim and Lee 1999, Kim 2005). Neosiphonia is characterized by having lateral branch initials and trichoblasts produced on successive segments, rhizoids cut off from pericentral cells by a cross wall, procarps bearing a three-celled carpogonial branch, spermatangial branches arising as a furcation of the trichoblasts, and spirally arranged tetrasporangia (Kim and Lee 1999, Kim 2003).
Recently, new combinations were made for Neosiphonia upolensis (Grunow) Kim et Boo from Malaysia (Kim et al. 2008), N. bajacali (Hollenberg) Mamoozadeh et Freshwater from Caribbean Mexico and N. echinata (Harvey) Mamoozadeh et Freshwater from Florida, USA (Mamoozadeh and Freshwater 2011), and N. sertularioides (Grateloup) Nam et Kang from Geojedo, Korea (Nam and Kang 2012). In the most recent study, Bustamante et al. (2012) reported a new species, N. peruviensis Bustamante, Won, Ramirez et T. O. Cho from Pisco, Ica, Peru.
Four Neosiphonia species are currently recognized from the Pacific temperate coast of South America: N. flaccidissima (Hollenberg) Kim et Lee; N. peruviensis Bustamante, Won, Ramirez et Cho; N. savatieri (Hariot) Kim et Lee; and N. sphaerocarpa (Børgesen) Kim et Lee (Howe 1914, Dawson et al. 1964, Ramirez and Santelices 1991, Bustamante et al. 2012). These species are characterized by rhizoids cutting off pericentral cells by a cross wall, ecorticate axes, and spirally arranged tetrasporangia.
We collected unidentified samples from Peru in 2008 and 2012 which we here describe as new species, Neo-siphonia ramirezii sp. nov., on the basis of their morphology. We also provide evidence of their phylogenetic relationship with other similar species, using comparative rbcL sequence analyses.
Samples were collected at Lagunillas, Pisco, Peru on August 2008 and July 2012, preserved in 4-5% formalin / seawater for the morphological study and dried in silica gel for the molecular study. Microscope observations were made on material stained with 1% aqueous aniline blue acidified with 0.1% diluted HCl. Photomicrographs were taken on an Olympus microscope (BX51TRF; Olympus, Tokyo, Japan) with an Olympus DP71 camera. A total of 25 individuals from 5 tufts were selected for measuring quantitative characters. Voucher specimens were deposited in the herbarium of Chosun University Korea (CUK).
Genomic DNA was extracted from silica gel-dried samples using the G-spin Iip genomic DNA extraction kit (iNtRON Biotechnology, Inc., Seongnam, Korea). The rbcL gene was amplified using the primer combinations F7-R753 and F645-Rrbcst, as listed in Lin et al. (2001), and was sequenced with the primers F7, F645, F993, R376, R753, R1150, and RrbcStart (Freshwater and Rueness 1994, Lin et al. 2001, Gavio and Fredericq 2002). PCR and sequencing protocols were as described in Cho et al. (2003). Sequences were determined for both forward and reverse strands using an ABI Prism 3100 Genetic Analyzer (PE Applied Biosystems, Foster City, CA, USA). New rbcL sequences were obtained from N. ramirezii and have been deposited in EMBL / GenBank under the accession numbers KC493351 and KC493352. All rbcL sequence data were compiled and the sequences aligned in the Genetic Data Environment (GDE 2.2) program (Smith et al. 1994). Phylogenetic analyses were conducted using MEGA version 5 (Tamura et al. 2011). Support for nodes in the maximum parsimony tree was determined by calculating 500 bootstrap proportion replicates (Felsenstein 1985). For maximum likelihood, we performed a likelihood ratio test using Modeltest 3.06 (Posada and Crandall 1998) to determine the best available model for the rbcL data. Maximum likelihood analyses were conducted using the GTR + I + G model, with 500 bootstrap replicates.
Diagnosis. Plants 2.4-5 cm high, saxicolous, consisting of limited prostrate and well-developed erect systems; branches dichotomous, attached by abundant unicellular rhizoids. Pericentral cells four, ecorticated along the entire thallus. Trichoblasts scarce, simple or forked once. Rhizoids cut off from pericentral cells by cross walls along the thallus. Procarps with three-celled carpogonial branches. Spermatangial branches arising from basal cells of forked trichoblasts. Tetrasporangia tetrahedral, spirally arranged.
Holotype. Lagunillas, Pisco, Ica, Peru, collected by T. O. Cho, Aug 21, 2008, CUK 6511 (Fig. 1A).
Other specimens examined. Lagunillas, Pisco, Ica, Peru, collected by T. O. Cho, Aug 21, 2008, CUK 6520; Lagunillas, Pisco, Ica, Peru, collected by T. O. Cho and D. E. Bustamante, Jul 5, 2012, CUK 8360.
Etymology of specific epithet. The new species honors Maria Eliana Ramirez for her pioneering and valuable contributions to the understanding of marine algae from the Pacific temperate coast of South America.
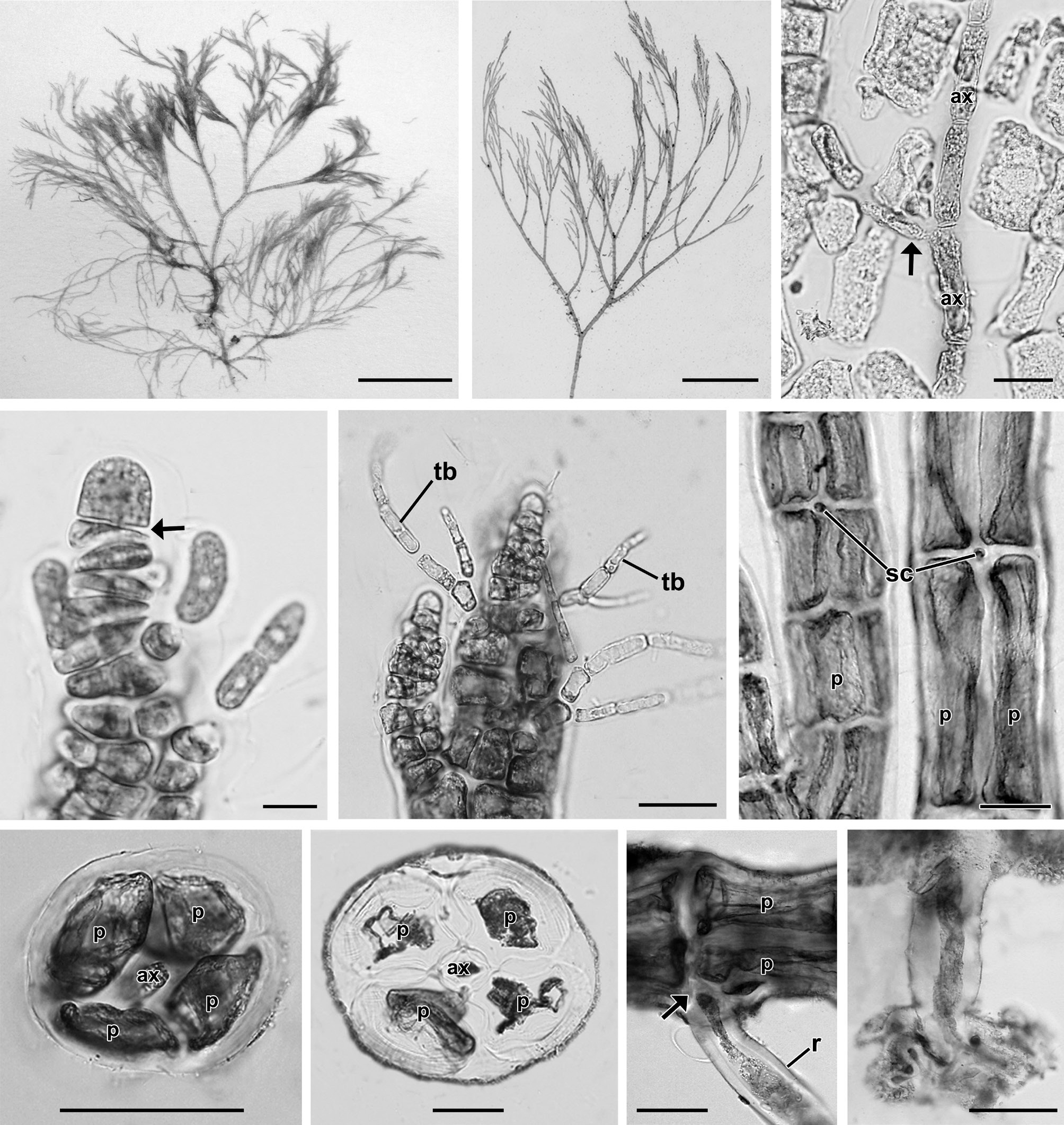
Description. Plants grow in the intertidal zone on rocks in association with other filamentous species. Thalli are 2.4 to 5 cm high (Fig. 1A), red-brown in color and occur in dense tufts (Fig. 1B) consisting of a limited prostrate system and well-developed erect filaments. Erect axes are 95.3 ± 9.8 μm in diameter (Fig. 1B) and arise endogenously from prostrate axes (Fig. 1C). Apices have a prominent apical cell, 8.2 ± 1.4 μm × 6.9 ± 1 μm in size (Fig. 1D). Trichoblasts are 22.7 ± 8.2 μm in length, deciduous, delicate, and scarce, produced from each segment near the apex, and once or twice forked (Fig. 1E). Inconspicuous scar cells are 11.8 ± 3.9 μm × 11.9 ± 3.9 μm, visible on the surface after shedding of trichoblasts (Fig. 1F). Adventitious branchlets arise on basal and older segments of the main axes as well as on its dichotomies. Four pericentral cells remain completely ecorticated throughout the thallus (Fig. 1G & H). Segments of erect branches measure 136.4 ± 43.6 μm in length and are 0.7 broader than long (1.4 ± 0.4 in length/breadth). Prostrate axes are 168.5 ± 11.1 μm in diameter and are attached on the surface of rocky substrata by numerous unicellular rhizoids located in the proximal and distal parts of the pericentral cells. Segments of prostrate axes are 279.4 ± 24.8 μm in length and 0.6 broader than long (1.7 ± 0.2 in length/breadth). Branching pattern is dichotomous in the main axes (Fig. 1B)
[Fig. 1.] Vegetative structures of Neosiphonia ramirezii sp. nov. (A) Holotype specimen from Lagunillas, Pisco, Peru, CUK 6511. (B) Habit of vegetative plant showing dichotomous branching pattern and pseudofastigiate appearance, CUK 6511. (C) Axial filament (arrow) endogenously developed from main axial cells (ax), CUK 6511. (D) Apical region of thallus showing a prominent apical cell with transverse division (arrow) of subapical cell, CUK 8360. (E) Apical region with trichoblasts (tb), CUK 8360. (F) Middle part of thallus with scar cells (sc), CUK 6511. (G & H) Cross section views of upper (G) and basal (H) parts of thallus (ax, axial cell; p, pericentral cell), CUK 8360. (I) Rhizoid (r) cut off from pericentral cell (p) by a wall (arrow), CUK 6511. (J) Unicellular rhizoid with digitate tips, CUK 8630. Scale bars represent: A, 1 cm; B, 5 mm; C & E, 20 μm; D, 5 μm; F-J, 50 μm.
[Fig. 2.] Reproductive structures of Neosiphonia ramirezii sp. nov. (A) Female gametophyte bearing cystocarps, CUK 8630. (B) Apical part of thallus with cystocarps (arrows) at various stages of development, CUK 8630. (C & D) Young (C) and mature procarp (D) with a three-celled carpogonial branch and trichogyne (tr), CUK 8630. ax, axial cell; cb1-cb2, sequence of carpogonial branch cells; cp, carpogonium; p, pericentral cell; su, supporting cell. (E) Male gametophyte, CUK 8630. (F) Apex showing spermatangial branch (arrow) developed on the basal cell of trichoblast (tb), CUK 8630. (G) Tetrasporangial plant, CUK 6511. (H & I) Cross section view of fertile segment (H) showing 5 pericentral cells, and mature stage (I) showing one tetrasporangium (t) surrounded by cover cells (arrows), CUK 6511. (J) Upper part of tetrasporangial thallus showing interrupted and spiral series of mature tetrasporangia (t), CUK 6511. Scale bars represent: A, 200 μm; B, F, H & I, 50 μm; C & D, 10 μm; E & G, 5 mm; J, 100 μm.
In female plants (Fig. 2A), cystocarps are narrowly globose, ovate, 152 ± 50 μm × 175 ± 57 μm in size, scattered on branchlets (Fig. 2B), and produced on short pedicels. Procarps consist of three-celled carpogonial branches (Fig. 2C & D). In male plants (Fig. 2E), spermatangial branches each arise from the basal cell of a forked trichoblast (Fig. 2F). In tetrasporangial plants (Fig. 2G), the fertile segments comprise 5 pericentral cells (Fig. 2H). Tetrasporangia are tetrahedral, swollen, distorted, and 59.8 ± 13 μm × 87.8 ± 18.4 μm in size. A single tetrasporangium is produced per single segment (Fig. 2I). Tetrasporangia are spirally arranged (Fig. 2J) with successively maturing sporangia.
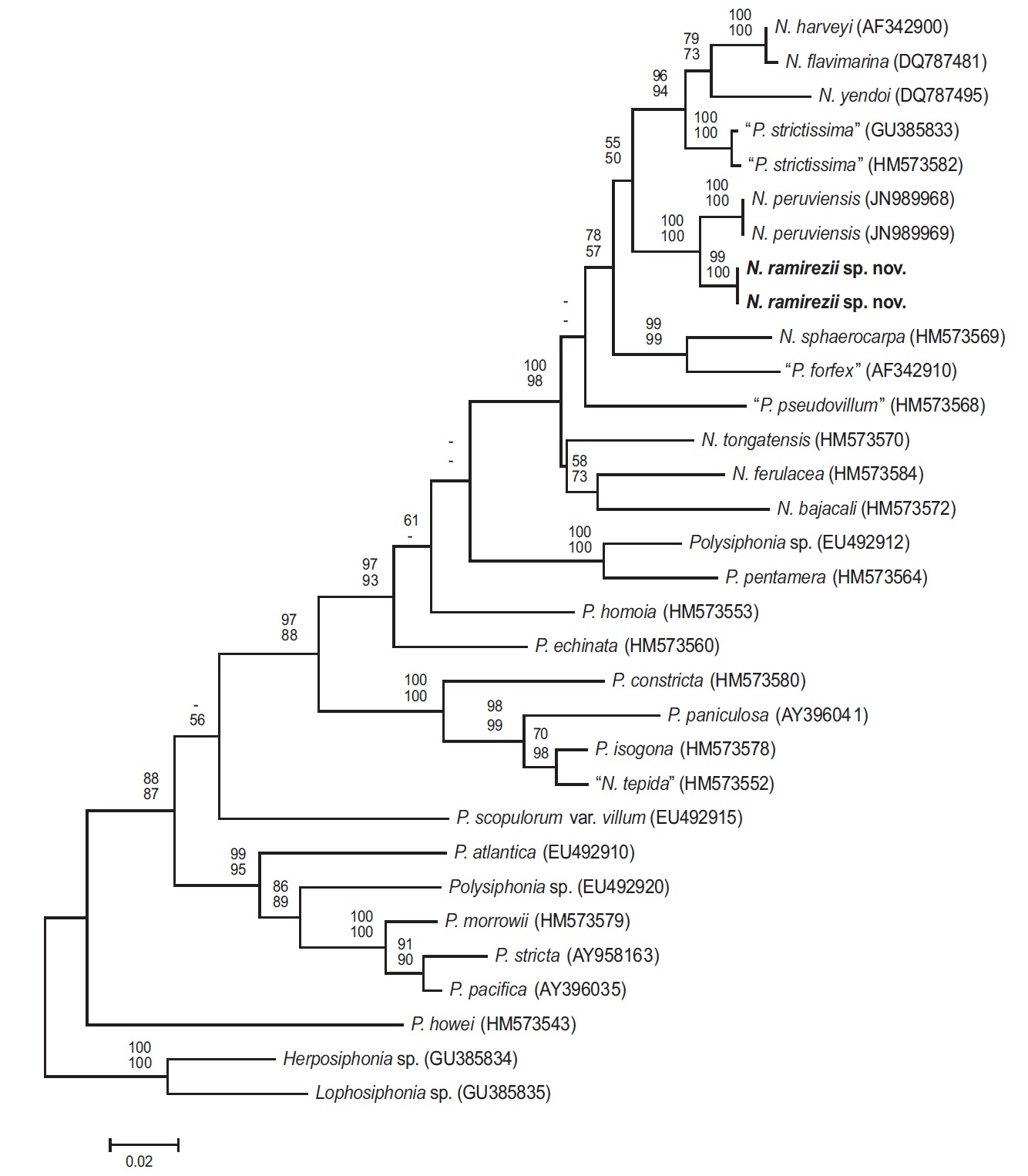
Phylogenetic analyses. The 1,244-bp portion of the 1,467-bp rbcL gene (84.8% sequenced) was analyzed, includes 347 parsimony informative sites. The phylogenetic trees were obtained from the alignment of the rbcL sequences which were newly generated and downloaded from GenBank. Herposiphonia sp. and Lophosiphonia sp. were selected as an outgroup taxa. Phylogenetic analyses reveal a strongly supported clade of predominantely Neosiphonia species in the rbcL maximum likelihood phylogeny (Fig. 3), with N. ramirezii located within this strongly supported clade, sister to N. peruviensis. The rbcL sequence divergence (%) for N. ramirezii specimens differed from N. peruviensis by 2.4%, from “Polysiphonia strictissima” by 5.2%, from N. yendoi by 7.0%, from N. flavimarina by 6.3 %, and from N. harveyi by 6.2% sequence divergence.
Neosiphonia ramirezii sp. nov. is newly described from Peru based on morphological and molecular evidence. It is recognized by the presence of erect filaments arising from limited prostrate filaments, numerous rhizoids cut off from irregular positions of pericentral cells by a cross wall, 4 pericentral cells throughout, ecorticate axes, scarce trichoblasts, inconspicuous scar cells, procarps with three-celled carpogonial branches, spermatangia arising from the basal cell of trichoblasts, and a spiral arrangement of tetrasporangia. RbcL molecular analysis corroborates the taxonomic placement of N. ramirezii in Neosiphonia.
Four Neosiphonia species have been reported from the Pacific temperate coast of South America: N. flaccidissima, N. peruviensis, N. savatieri, and N. sphaerocarpa. Although these Neosiphonia species co-occur with our new species in the Pacific temperate coast of South America, they are morphologically distinct from N. ramirezii sp. nov. N. flaccidissima reported from Lima, Peru, is distinguished by having branches arising in connection with trichoblasts, indistinct main axes, and abundant trichoblasts (Hollenberg 1942, 1968, Dawson et al. 1964). N. peruviensis described from Ica, Peru, has 6 pericentral cells and 1 or 2 tetrasporangia per segment (Bustamante et al. 2012). N. savatieri reported from Isla de Pascua, has a rhizoidal cluster and abundant trichoblasts (Hariot 1891, Santelices and Abbott 1987, Kim 2005). N. sphaerocarpa, reported from Lima and Talara, Peru, is distinguished by having creeping prostrate filaments and abundant trichoblasts with 3-4 dichotomies (Børgesen 1918, Dawson et al. 1964, Hollenberg 1968, Hollenberg and Norris 1977, Guimaraes et al. 2004, Mamoozadeh and Freshwater 2011).
N. ramirezii sp. nov. resembles the other 14 worldwide Neosiphonia species (Harvey 1853, Kutzing 1863, Hollenberg 1942, 1961, 1968, Kapraun 1977, Maggs and Hommersand 1993, Womersley 2003, Guiry and Guiry 2013) by having 4 pericentral cells, ecorticate axes, exogenous lateral branches, procarps with three-celled carpogonial branches, spermatangia developed from the basal cell of a forked trichoblast, and spirally arranged tetrasporangia (Table 1). However, the species (symbol a) labeled in Table 1 are distinguished from N. ramirezii by the presence of a discoidal base or rhizoidal cluster (Harvey 1853, Segi 1951, Hollenberg 1968, Kapraun 1979, Yoon 1986, Kim and Lee 1999, Guimaraes et al. 2004, Kim and Abbott 2006, Kim et al. 2008, Mamoozadeh and Freshwater 2012). Furthermore, the species (symbol b) labeled in Table 1 are distinct from N. ramirezii by having creeping filaments or extended prostrate filaments (Agardh 1863, Hollenberg 1942, 1968, Segi 1951, Kim and Lee 1996, 1999, Abbott et al. 2002, Womersley 2003, Kim and Abbott 2006, Kim et al. 2008, Lee 2008, Creed et al. 2010, Nam and Kang 2012). The remaining species in Table 1, N. apiculata (Hollenberg) Masuda et Kogame, is characterized by abruptly tapered apices and pseudodichotomous branches (Hollenberg 1968, Tani et al. 2005, Kim and Abbott 2006).
Our molecular phylogenetic analyses using rbcL sequences revealed sequence divergence between N. ramirezii sp. nov. and other previously reported Neosiphonia species, sufficient to classify our new species among Neosiphonia. Neosiphonia ramirezii is nested in a monophyletic, well-supported clade.